Atomic Structure and Radiation
What we are learning:
Atoms:

Let's start off with how big, or rather how small atoms are. The radius of an atom is around 1 x 10-10m. Despite this being a tiny distance, the nucleus's radius is about 1/10000 of the whole atom. In chemistry you will have already looked at the structure of the atom, however, in chemistry we are interested more in the interactions of the electrons as they form ions and bonds, in physics, we focus more on the nucleus. In the nucleus, there are 2 particles or nucleons, they are the positive protons and the neutral neutrons. The overall charge is balanced out by the negatively charged negative electrons. These electrons are orbiting at different distances from the nucleus, these are the different shells that you have met in chemistry.
Just like in chemistry, the atomic number is the number of protons in the nucleus, this is what defines the elements. Cobalt atoms may have a variety of numbers of neutrons (isotopes) but they must all have 27 protons to make them cobalt. The atomic mass is simply the number of nucleons (protons and neutrons added together). For atoms, the numbers of protons and electrons are equal, however, for a charged ion, there is an imbalance, metals lose electrons and form positive ions and non-metals gain electrons and form negative ions.
Atomic models over time:

You have already learned about the models of the atom and how they have changed over time following new evidence from experimentation. Please go back and read the section labelled the model of the atom.
Radioactive decay:

Before we delve into this fascinating part of physics, let's get one thing perfectly clear from the start, in radioactivity and decay, we will talk about a nucleus, a collection of nuclei but never atoms. Only nuclei decay, not atoms. Nice to get that off my chest, now onto business.
When a nucleus is unstable, it needs to stabilise itself and to do this, it undergoes nuclear decay. Apologies in advance for the following, but it makes sense to me. If you went out for the day and you bought a dodgy kebab or some iffy shellfish, your stomach would grumble and gurgle (you are now unstable). In order to stabilise your body will quickly "eject" the offending item and you feel better. With an unstable nucleus, it will eject 1 of the 3 types of nuclear radiation.
The process is completely random and there is no way to predict which of the nuclei will decay next. The number of decays per second are called Becquerels (Bq).
Types of nuclear radiation & their properties:

There are three types of nuclear radiation.
Alpha (α):
This is the largest, most ionising type. It is also the most easily stopped and can travel the least distance in air. It is a helium nucleus or 2 protons and 2 neutrons. This means that it has a mass of 4 and a charge of 2+. It is stopped by paper, can only get a couple of cm in air. Luckily for us, although it is the most ionising, it cannot get through our skin (breathing in or ingesting α sources can be lethal). Alpha is used in smoke alarms, if smoke particles get between the source and the detector, it will stop the alpha particles and set off the alarm.
Beta (Β):
During beta decay, a neutron changes into a proton and a high speed electron. The beta particle is the electron that is fired out of the nucleus. It is smaller than alpha so can travel further (1 m in air) and is stopped by thin aluminium. As the charge is 1-, it is less ionising than alpha. Beta is used to measure the thickness of aluminium foil, as the foil gets too thick, fewer particles are counted passing through the foil and if the foil gets too thin, then the count goes up.
Gamma (γ):
Gamma is not a particle, it is an electromagnetic wave as the nucleus ejects excess energy in this form. It has no charge so is the least ionising, however, it takes thick lead or metres of concrete to absorb it and in space, it has an infinite distance. It is used in both cancer treatment and to sterilise hospital equipment on its way into an operating theatre.
Neutron:
This final particle is released during nuclear fission. Here, an unstable nucleus absorbs a slow moving neutron then splits into 2 similarly sized daughter nuclei and more neutrons. This is the process that occurs in a nuclear power station.
Decay equations:
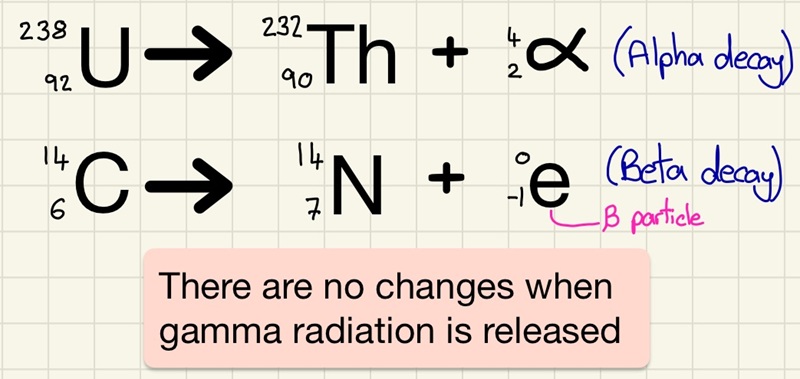
When an unstable nucleus undergoes decay and it releases either an alpha particle or a beta particle, then there are changes to it that mean that the atomic mass and/or atomic number need to be updated.
In an alpha decay, we lose 2 protons and 2 neutrons. This means that the original atom will lose 2 from the atomic number and 4 from the atomic mass.
In a beta decay, a neutron becomes a proton and a high speed electron. This means that the original atom will gain 1 on the atomic number and the atomic mass will remain the same.
If gamma radiation is emitted, there is no change.
Half life:
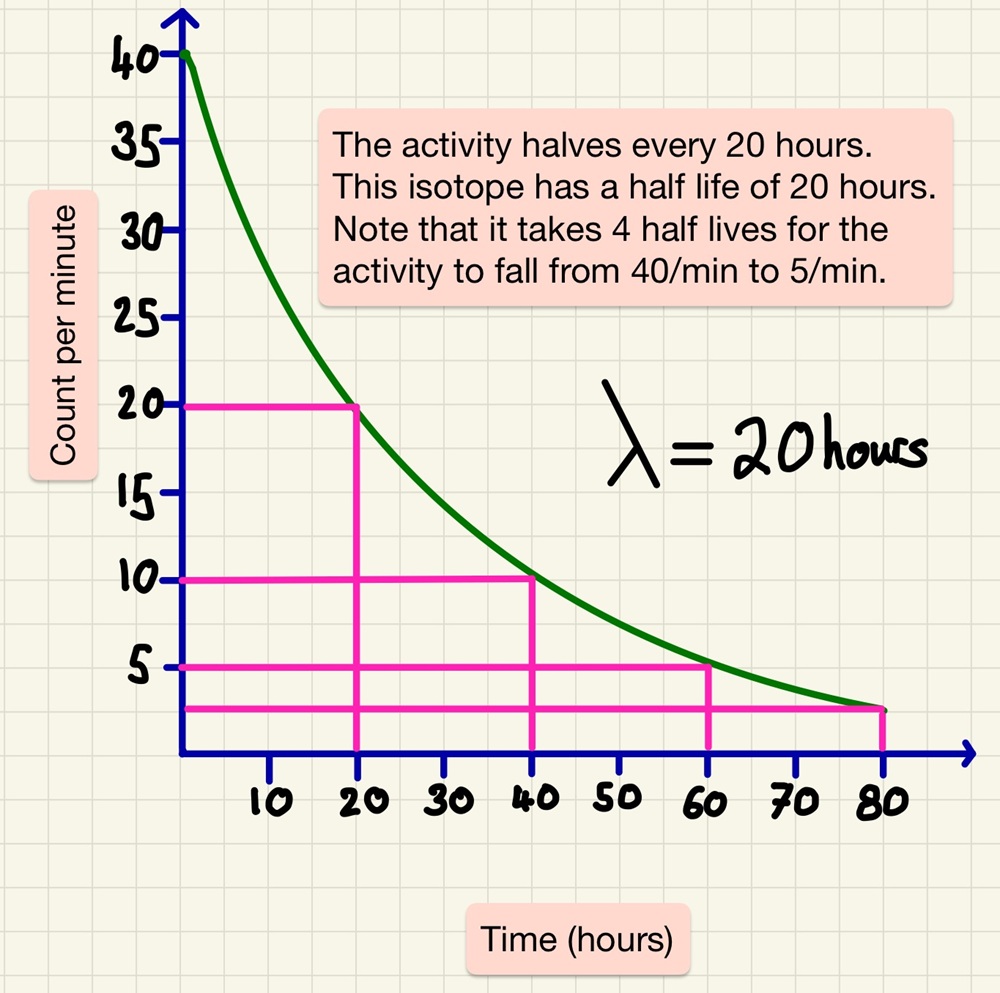
Half life is the time it takes for half of the unstable nuclei in a sample to decay or the time it takes for the activity (Bq) to halve. Although it is impossible to predict when a particular unstable nucleus will decay, the half life of an isotope of an element is extremely predictable. So much so that we can calculate how long a sample has been in existence. After one half-life, the activity halves, after another half-life has passed, it halves again (now meaning that the activity is a quarter of the original). This data is easy to see on a graph and you should be able to identify every time that the activity halves.
Contamination & irradiation:

These two terms mean different things though people often mistakenly interchange them.
Contamination is the terms used to describe when a person or an item has unwanted radioactive materials land on it or stick to it. This may be from a radioactive dust that can land on surfaces. In schools, there may be radioactive samples to be used in demonstrations, these are only picked up with tweezers and the radioactive material is encased in a metal cup to prevent actual contact. These samples are checked regularly in case fragments begin to flake off as these will become a source of contamination. Alpha sources are particularly dangerous as if the dust is breathed in, there is no skin to protect us and the highly ionising nature will cause great harm.
Irradiation is when an item is exposed to ionising radiation. Some things may not be bothered by this but if people are irradiated, it may cause harm. Objects do not become radioactive themselves when irradiated. Irradiation is used to kill microorganisms in foods and on surgical equipment just before entering an operating theatre.
Key words/terms for this topic
Activity Alpha Decay Atomic Models Beta Decay Contamination Electrons Gamma Radiation Half Life Irradiation Isotope Neutrons Nucleon Nucleus Plumb Pudding Model Protons Radioactivity Scattering Experiment Tangent
Curriculum Health Check:
Q: What is the term to describe the time taken for half of the unstable nuclei to decay? It is also the time taken for the activity of a sample to halve.
A: Diminished-life
B: Half-life
C: Stability time
D: Decay time
What you need to know
The structure of atoms is split between the positive nucleus (positive protons and neutral neutrons) and the negative electrons orbiting in shells. Chemists focus more on the electrons whereas physicists focus more on the nucleus. Atoms have a radius of about 1 x 10-10m.

Link to chemistry's structure of the atom and how it links to the numbers on the periodic table. The other common part from chemistry is the development of the theory of the model of the atom. Ensure that you can describe the scattering experiment.

Understand the different types of nuclear radiation, α, β and γ radiation. You need to know how they are produced, how far they travel in air, what materials can stop them, how dangerous they are and what we use them for.

Describe how activity is measured and the unit becquerel (Bq).

Use nuclear equations that will balance to show original isotope, the particle that has been emitted and the new isotope (daughter).

Describe nuclear half life.

Understand and describe both radioactive contamination and irradiation. They can both be created in a similar situation, however, they are different and you need to distinguish clearly. List hazards associated with both, suitable precautions and how they affect people, animals and plant life.

Extra topics needed for the Higher Tier papers:

As well as describing half life, you need to be able to do this graphically as well as sing a data table. You may be asked to calculate the activity after 3 half lives have passed or find out the half life from data showing a decay in activity.

This page was updated on: 2nd April 2024

