Chemical Analysis
What we are learning:
Pure substances and how to test purity

A pure substance must be made from a single element or a single compound. Outside the world of chemistry, the word pure is used differently and can be misleading. If you look at the ingredients list on a carton of "pure orange juice" you will see that it is made of many different compounds.
Pure substances can be tested by melting or boiling them. A pure substance will melt or boil at a precise point whereas a mixture will have a melting or boiling range.
Formulations:

A formulation is a precise recipe of different elements and compounds to complete a particular task. Every ingredient must do a specific job and the components must not react with each other. These formulations have likely been tweaked and adjusted over time to be specific for that task. There are many examples of these including: paints, fertilisers, medicines, fuels, cleaning products, food stuffs and alloys. An example of the precision needed is when you buy paint to decorate a room, if you buy 4 cans that have been made at different times, they need to react the same on your walls, they need to be the same colour and they need to age the same over time.
Chromatography:
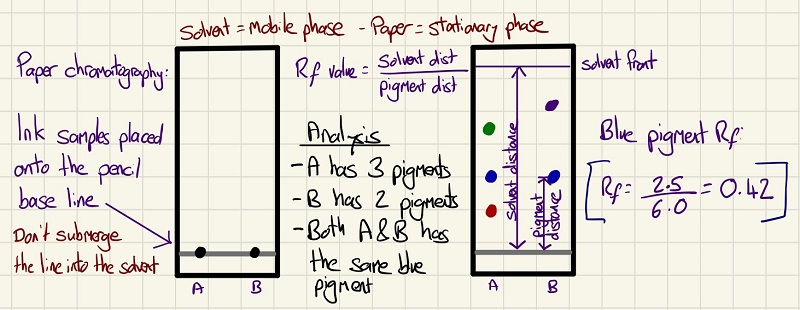
Chromatography is a method of separating substances by their solubility in the solvent. By setting up the experiment, the pigments will dissolve in the solvent and as the solvent moves up the paper through capillary action, the pigments are taken with it until the pigment is no longer soluble and it appears on the paper. The more soluble the pigment, the higher up it travels. If a pigment does not leave the start line, it is insoluble in that solvent. You need to be able to work out the Rf value of the pigment to compare it with other chromatograms. You will need to have a go yourself as this is a required practical.
Testing for gases:
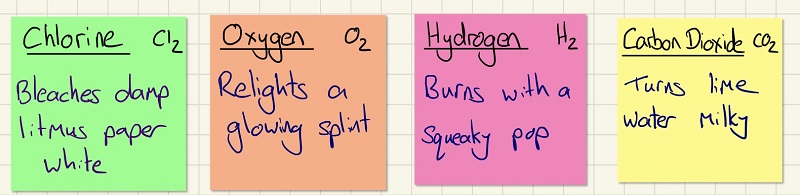
This is a very simple part and you need to be able to remember the 4 tests for gases. The test for:
CO2 = turns limewater milky,
H2 = burns with a squeaky pop,
Cl2 = bleaches damp blue litmus paper white,
O2 = relights a glowing splint.
Key words/terms for this topic
Chromatography Formulation Gas chromatography Impure Mobile phase Pigment Pure Purity Rf value Retention time Solute Solution Solvent Solvent front Stationary phase
Curriculum Health Check:
Q: Why is knowing the Rf of pigments useful?
A: You can identify errors in the experiment
B: You can see how to make new colours
C: You can see what inks a pigment is made from
D: You can see if pigments are common across different inks
What you need to know
Pure substances contain only a single element or compound. Don't get these mixed up with commercial purity. Pure orange juice is not chemically pure because it contains lots of different compounds.

Formulations are precise mixtures such as medicines, paints, alloys and cleaning products.

Chromatography is used to separate pigments that are in inks and food colouring. The solvent used is the mobile phase and the paper is the stationary phase. You need to be able to calculate Rfvalues (divide the distance the substance moved by the distance the solvent moved) and compare which pigments are in a colouring/ink.

Required practical 12: Use chromatography to separate the pigments in a coloured substance. Calculate the Rfvalue of a range of pigments.

You need to be able to describe the tests for 4 common gases and state what a positive result for each is:

• Oxygen - relights a glowing splint

• Hydrogen - when lit produces a squeaky pop

• Carbon dioxide - turns limewater milky

• Chlorine - bleaches damp blue litmus white

This page was updated on: 22nd February 2024

