Atmospheric Chemistry
What we are learning:
The gases in our atmosphere:
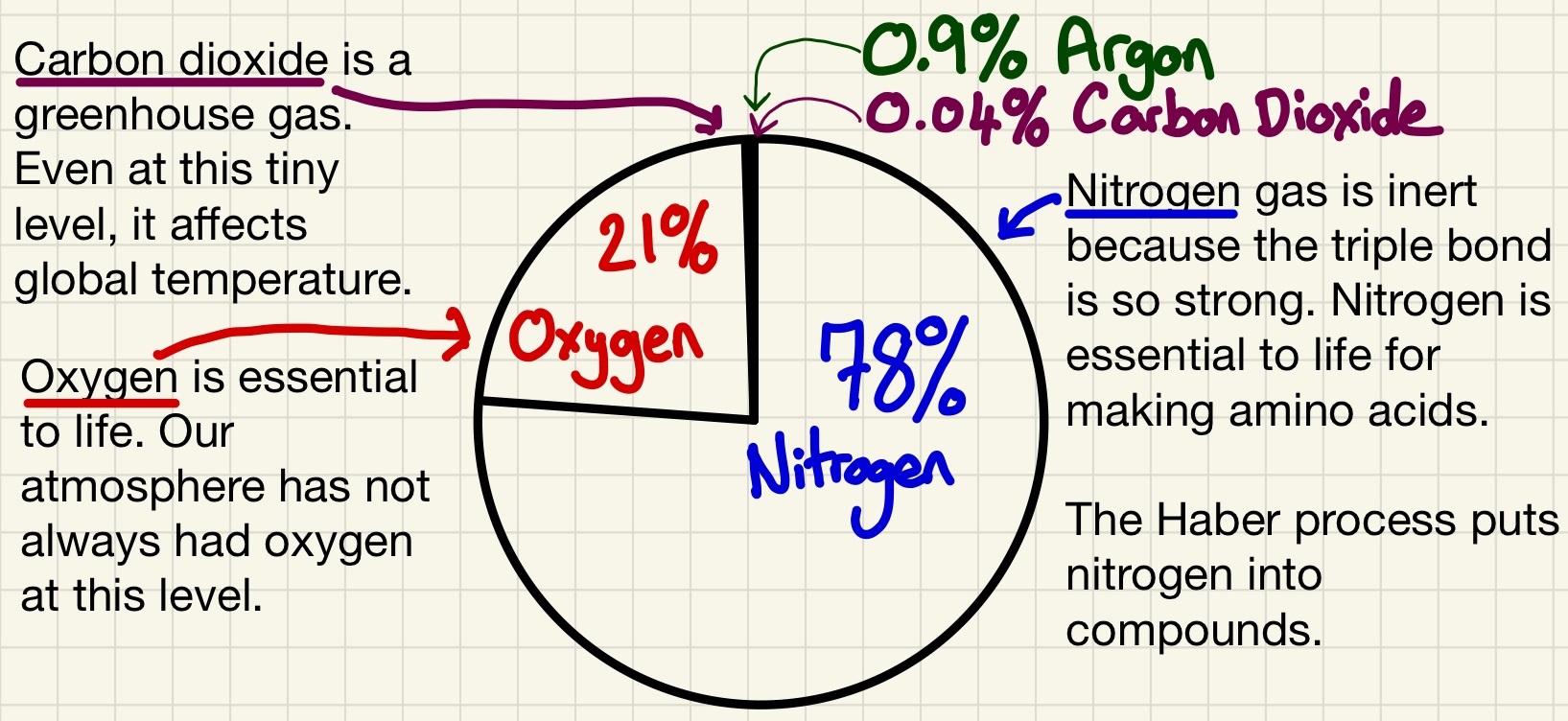
Our atmospheric gases and their relative proportions have been quite steady for the past 200 million years. We currently have an atmosphere that is 78% N2, 21% O2, 0.9% Ar and just 0.04% CO2. It is a good opportunity to recap the uses of these gases and to revisit the tests for gases as two of these you should know how to test for them.
The evolution of our atmosphere and those of other planets:
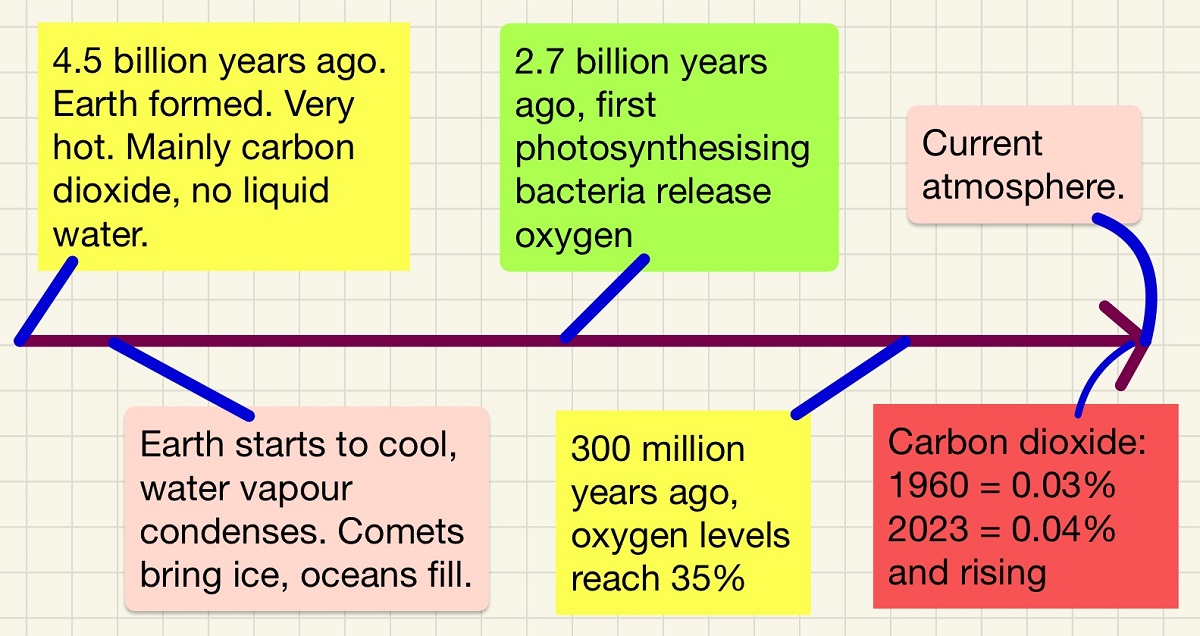
You need to understand how our atmosphere has changed over the 4.6 billion year life span of our planet and to know what drove those changes. Cooling caused the water vapour to condense and fall, volcanoes released huge amounts of carbon dioxide. This means that the early atmosphere was very much like the current atmospheres of Venus and Mars. Volcanoes also released large quantities of CO2, some of which dissolved in the oceans and gave us carbonate sediments. The next major change that set Earth apart from our planetary neighbours was life. Early life began to photosynthesise and most of the CO2 that remained in our atmosphere was broken down to sugars in the plant material and the waste product released was oxygen.
Although we have lots of evidence about this process, remember that nobody witnessed it and there are no rock deposits surviving from 4.6 billion years ago, this makes it reliant on theories based on evidence and these can and do change over time.
The atmosphere of Mars:

This is a great opportunity to look at the atmosphere of Mars by watching clips from "The Martian". It is a great film that shows some brilliant science that truly comes to life. Before going on, I strongly recommend that you read The Martian by Andy Weir. You get to see/read about: the atmosphere, extremes of temperature, growing crops, solar power, nuclear power, water treatment, catalysis, hydrazine, gravity and so many more things than I can begin to list.
The greenhouse effect and global warming:
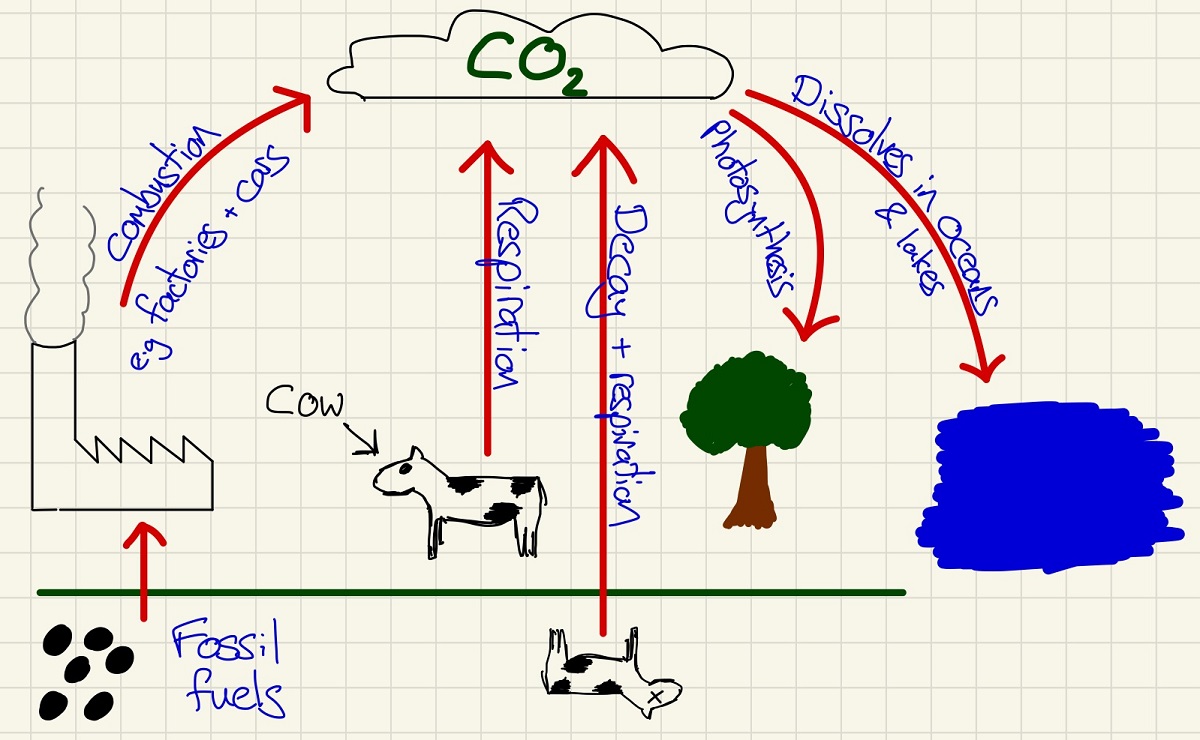
You will have met a basic version of this at the and of Key Stage 3. Here we can look into it in a little more detail. The greenhouse effect is NOT a bad thing and without it, Earth would be too cold to sustain life as we know it today. The carbon cycle shown tells us the problem - there are far more ways of adding carbon to the atmosphere than there are of removing it. When heat arrives from the sun as UV radiation (shortwave) it is absorbed by the Earth and at night, it is emitted as Infrared radiation (longwave). This Infrared radiation is absorbed by Carbon dioxide, methane and water vapour which retains heat in the atmosphere that would previously have escaped into space at night.
Carbon footprint and human impact:

There is no doubt that humans are having a negative effect on our atmosphere. There is an abundance of evidence that is peer reviewed and free from bias that proves we are increasing the average global temperature (global warming)by increasing the greenhouse effect. It is the release of more CO2 and methane that is unbalancing a very delicate system. As well as releasing mor of these gases, we are removing trees which is reducing the Earth's ability to redress the balance of atmospheric CO2. It is catastrophically dangerous when politicians claim that climate change is a myth because they are in a position of power and influence which makes the public more likely to believe them. It is crucial that young people all over the world are educated about the Earth's future being in their hands.
Everyone should be aware of their own carbon footprint and make decisions on their own purchases based on how it could add to or reduce their own impact on climate change.
Atmospheric pollutants:
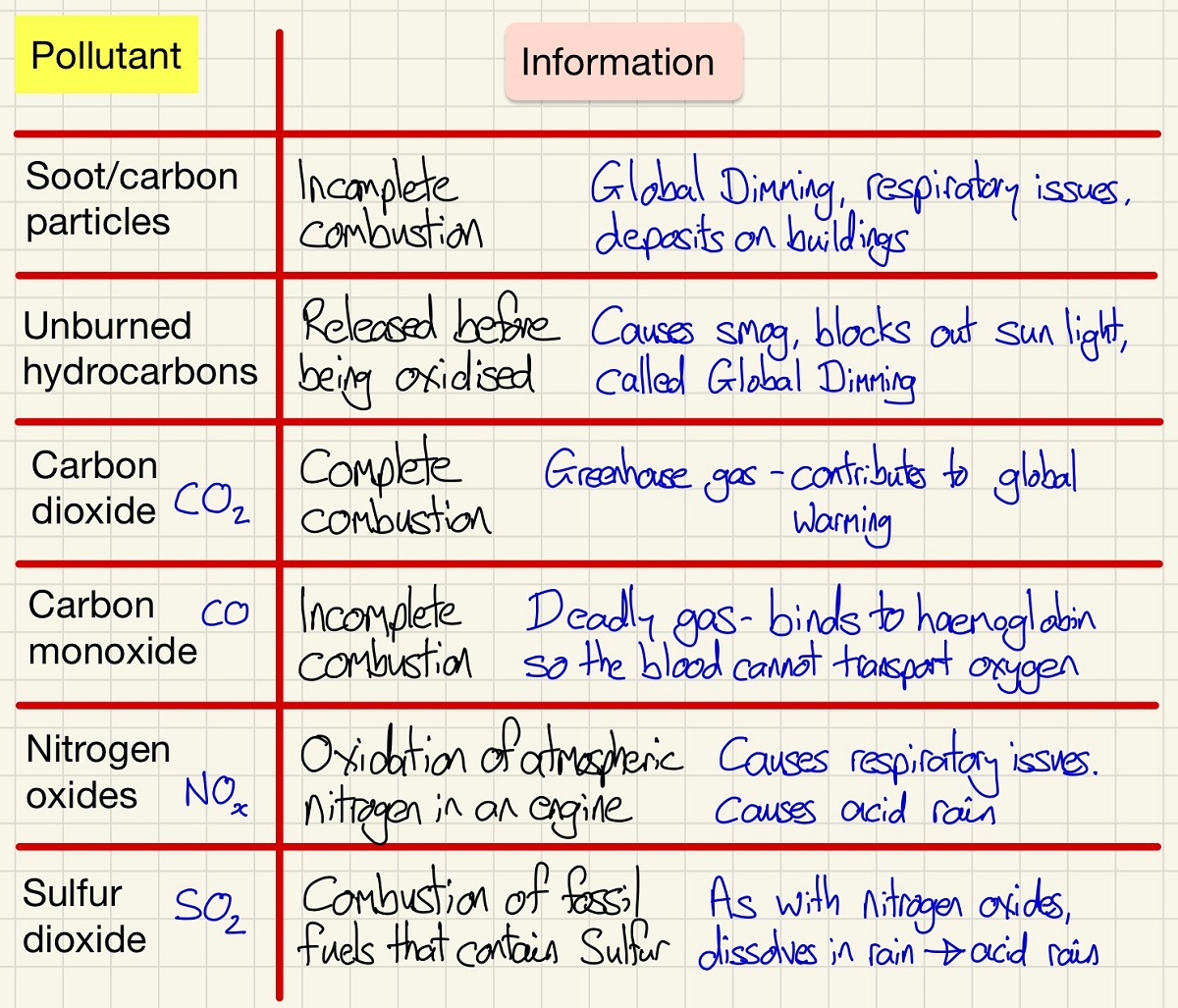
There are many different pollutants, some have a more immediate impact than others but all should be taken very seriously. Carbon Monoxide is the most important one to address, if you have any gas appliance at home/work, always ensure that it is fitted and serviced by a qualified Gas Safe engineer. When you go on holiday, if there are gas appliances, check that they are serviced properly and if in doubt, please please please invest in a carbon monoxide detector, it could literally save you life and those with you. If a gas appliance has soot or orange flames instead of blue - get it checked. It is not only gas appliances, anything that involved combustion - never bring a barbeque inside a tent/house.
The others that you need to know about are oxides of sulfur and nitrogen can produce acid rain, unburned hydrocarbons can produce smog and global dimming as they block out the sun.
Key words/terms for this topic
Acid rain Atmosphere Carbon cycle Carbon Footprint Carbon monoxide Carbon Sink Catalytic Converter Combustion Evolution Global Climate Change Global Dimming Global Warming Greenhouse Effect Greenhouse gas Particulate Photosynthesis Pollutant Pollution Stromatolite Terraforming
Curriculum Health Check:
Q: What is a carbon footprint?
A: The amount of any greenhouse gas an item or person is responsible for releasing in its lifetime
B: The amount of CO2 an item or person is responsible for releasing in its lifetime
C: The amount of methane an item or person is responsible for releasing in its lifetime
D: The amount of climate change an item or person is responsible for in its lifetime
What you need to know
The Earth's atmosphere over the past 200 million years has been 78% Nitrogen, 21% Oxygen and 1% Argon. There are others in tiny proportions like water vapour and other noble gases. Most important of these less common ones is carbon dioxide. This is about 0.04% of our atmosphere and rising.

Our atmosphere has evolved over the planet's 4.6 billion year life. We can predict what the early atmosphere was like based on the atmosphere's of Mars and Venus as well as analysing the gases that come out of volcanoes.

To begin with, the gases mainly came from volcanoes - Nitrogen and carbon dioxide with small amounts of methane and ammonia. CO2 dissolved into the oceans (formed from cooling water vapour and ice from many comets) which produced minerals such as calcium carbonate CaCO3 which produced rocks/sediments.

The first algae and early plants began the process of changing the atmosphere through photosynthesis. The huge amounts of CO2 were slowly converted (along with water needed for the process) to O2 and glucose in the presence of uv light. Since this began, approximately 2.7 billion years ago, animals have been able to live. You need to be able to describe the changes over time to get from our early atmosphere to the present day one.

The greenhouse effect is not a bad thing. Without it, Earth would be too cold. Greenhouse gases such as CO2, methane and water vapour trap in infrared radiation that is normally reflected out into space. Shortwave radiation from the sun gets through but lots of the longwave reflections are absorbed.

We have increased the greenhouse effect be releasing more CO2 and methane into the atmosphere. Mainly CO2 is a concern as it is released when we burn fossil fuels. This began to accelerate at the start of the industrial revolution and then again with population growth and cars. Climate change is real, however, it is difficult to convince many people, especially those who have a financial incentive to deny that it is happening. The average surface temperature increases as the percentage of CO2 increases. You may be expected to interpret graphs. You should also be aware of the predicted effects of climate change from rising sea levels, food shortages and extreme weather.

The carbon footprint of an item is the amount of CO2 and other greenhouse gases released in the full life of that product. You need to describe ways of reducing a carbon footprint.

Combustion of fuel produced atmospheric pollutants, there is sulfur in fossil fuels, these produce oxides of sulfur. Along with oxides of atmospheric nitrogen they can cause asthma and acid rain. Unburned hydrocarbons can cause global dimming. Carbon monoxide is a toxic gas that is not easily detected but is present in car exhaust gases. These all cause health problems for humans.

This page was updated on: 20th February 2024


