Chemical Changes
What we are learning:
Metal oxides:

Metals react with oxygen to form a metal oxide. Here you need to look at the word equations and the balanced symbol equations for these reactions. You need to look in depth at what is happening to the atoms and ions. The metals will lose their outer electrons to form positive ions, oxygen will gain 2 electrons and form a 2- ion. Both sets of ions will have full outer shells of electrons. This is mainly retrieval practice as you will have covered this in atomic structure.
Reactivity Series:

When metals react, we have seen that they form positive ions. Their tendency to form a positive ion (by ejecting their outer electrons and achieving a stable full outer shell) is how reactive they are. It is easier to say with how "much oomph" they get rid of those outer electrons. You do not need to know how every single metal fits into the hierarchy, but we need to remember some of the basics in this image. The most useful application is predicting displacement reactions. For example, as lithium is more reactive than copper, if copper chloride was mixed with lithium, lithium's level of "oomph" or reactivity is higher so it would become a positive ion and form a compound with chloring and poor old copper would become single again. Copper would be displaced by lithium.
The Blast furnace:
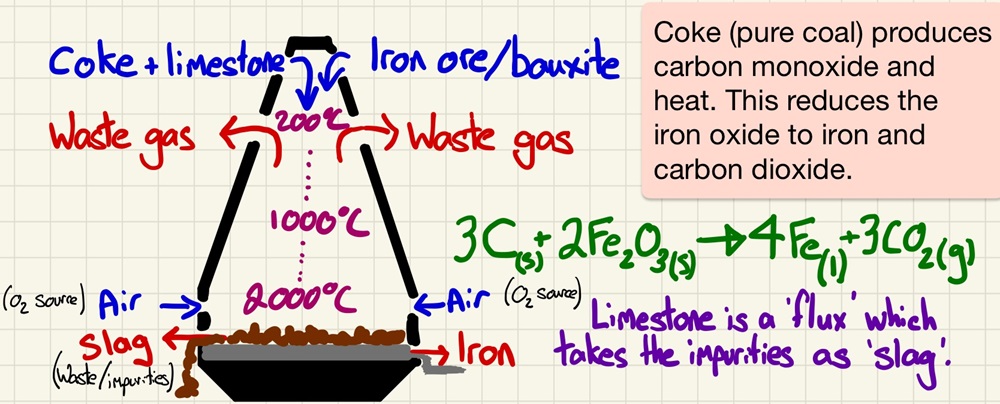
This is a great opportunity to look at chemistry in action and how this process of using a more reactive element (carbon) to remove the non-metal(s) from a less reactive metal (iron) in its ore has impacted life today. As well as the chemistry, you can look at the work of people like Abraham Darby and the Industrial Revolution. The effects of this chemical process impacted the whole world.
REDOX reactions:
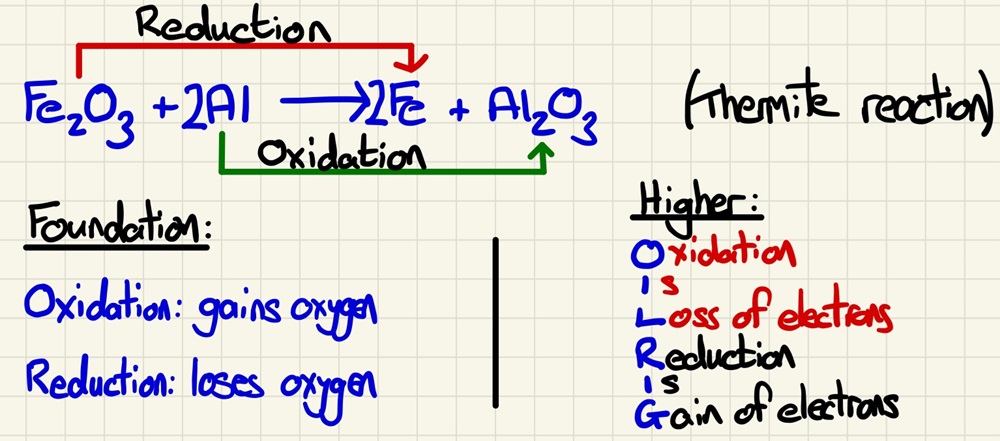
This follows on perfectly from the last lessons as the reaction in a blast furnace is a REDOX reaction as is any displacement reaction. REDOX comes from 2 words, Reduction and Oxidation. They are combined because one cannot happen without the other. Reduction is the removal of oxygen and Oxidation is the gaining of oxygen. On Higher Tier papers, we also look at the transfer of electrons. OILRIG is a way to remember that Oxidation Is Loss or electrons and Reduction Is Gain of electrons. Both of these show why they have to happen simultaneously, something must give the oxygen if something is to receive it, just as with the loss and gain of electrons.
Reactions of metals with acids:

Metals react with acids to produce salts and hydrogen gas. In actual fact, we have already seen that some metals are extremely unreactive so at room temperatures they don't react. You should be able to investigate how sulfuric acid reacts with Iron, Zinc and Magnesium. You will observe different levels of reactivity. If you are sitting the higher tier paper, you should be able to produce balanced symbol equations for all of the equations shown and be able to show that each of these is a REDOX reaction in terms of gaining and losing electrons.
Acids, Alkalis, Bases and pH:
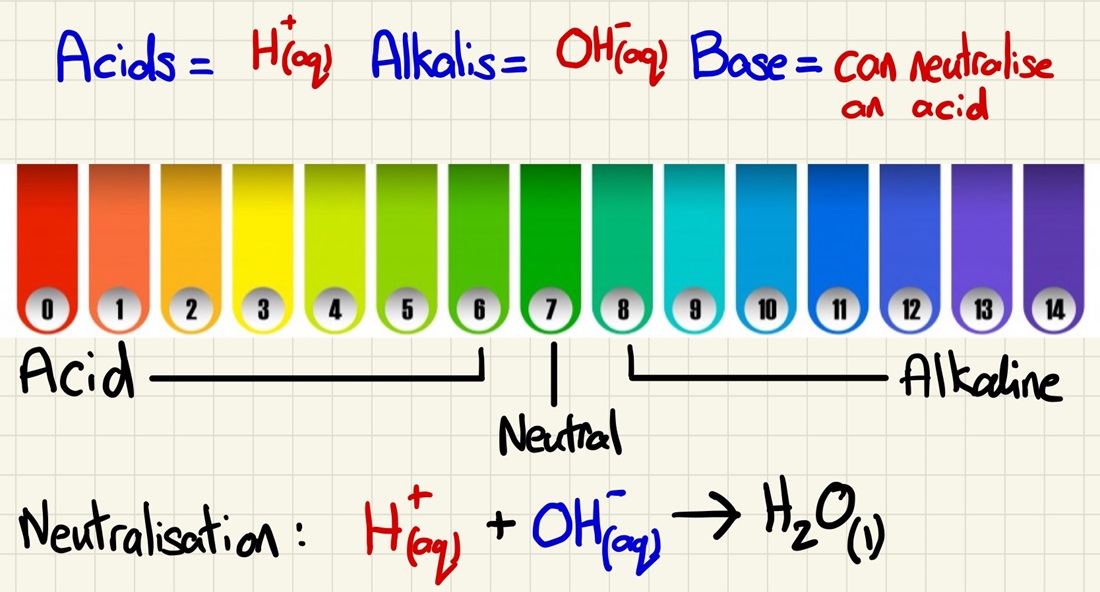
An Acid is a solution that has H+ ions and an Alkali is a solution that contains OH- ions. These are the fundamental things that make them acids or alkalis. A Base will neutralise an acid and is usually an insoluble metal hydroxide. This links very closely to the equations above.
The pH scale is simply a measure of the concentrations of either hydrogen ions (acid) or hydroxide ions (alkali). It is very important to note that it is a logarithmic scale, this means that a H+ solution with a pH of 3 is ten times more concentrated than a H+ solution with a pH of 4.
Neutralisation reactions:
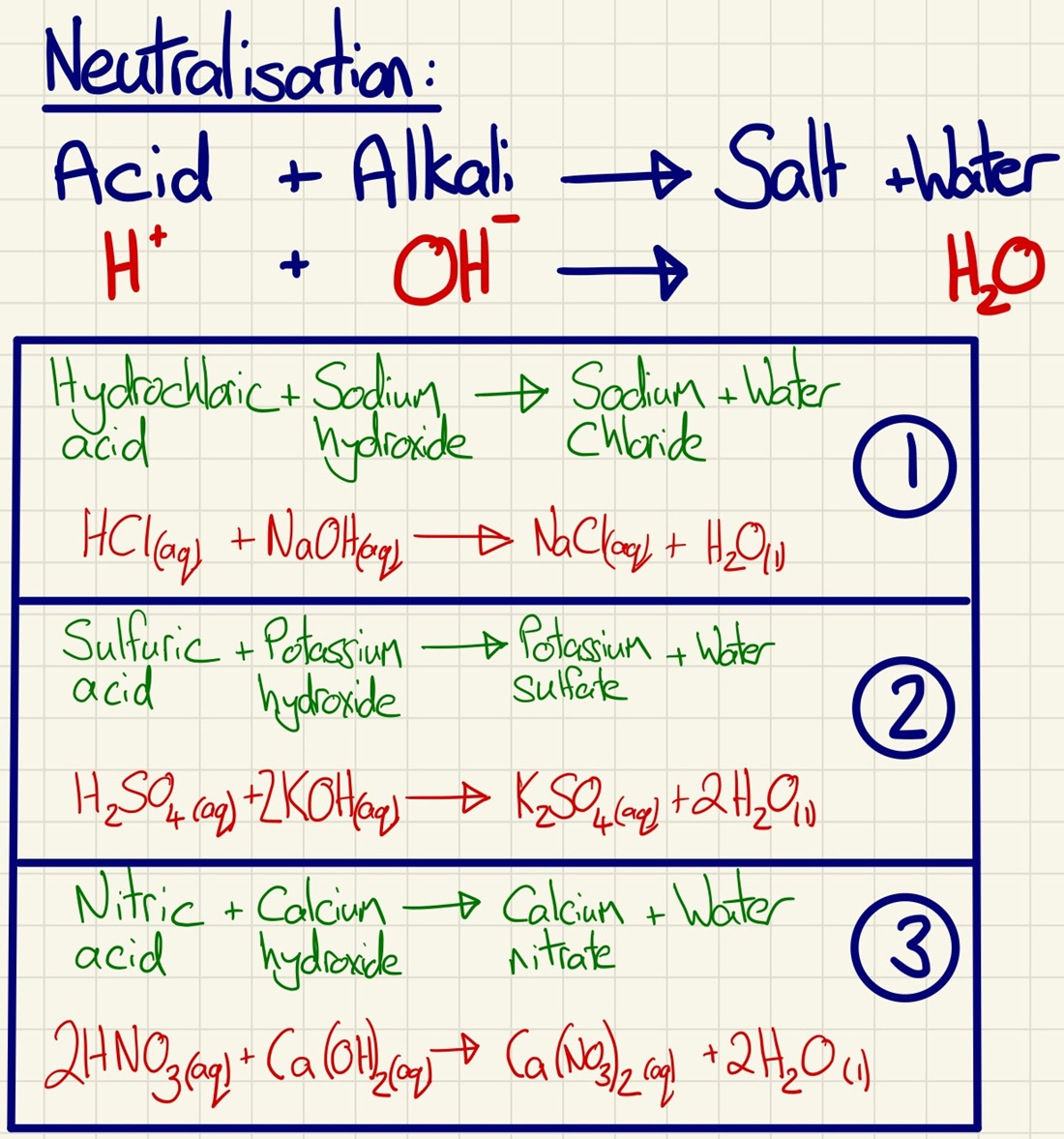
This component is about acids reacting with either an alkali or a base to form a neutral solution. This is an excellent opportunity to work on balancing equations and (HT) going back to the previous topic, quantitative chemistry, an looking again at concentrations and even calculating unknown concentrations from titrations.
Making salts:
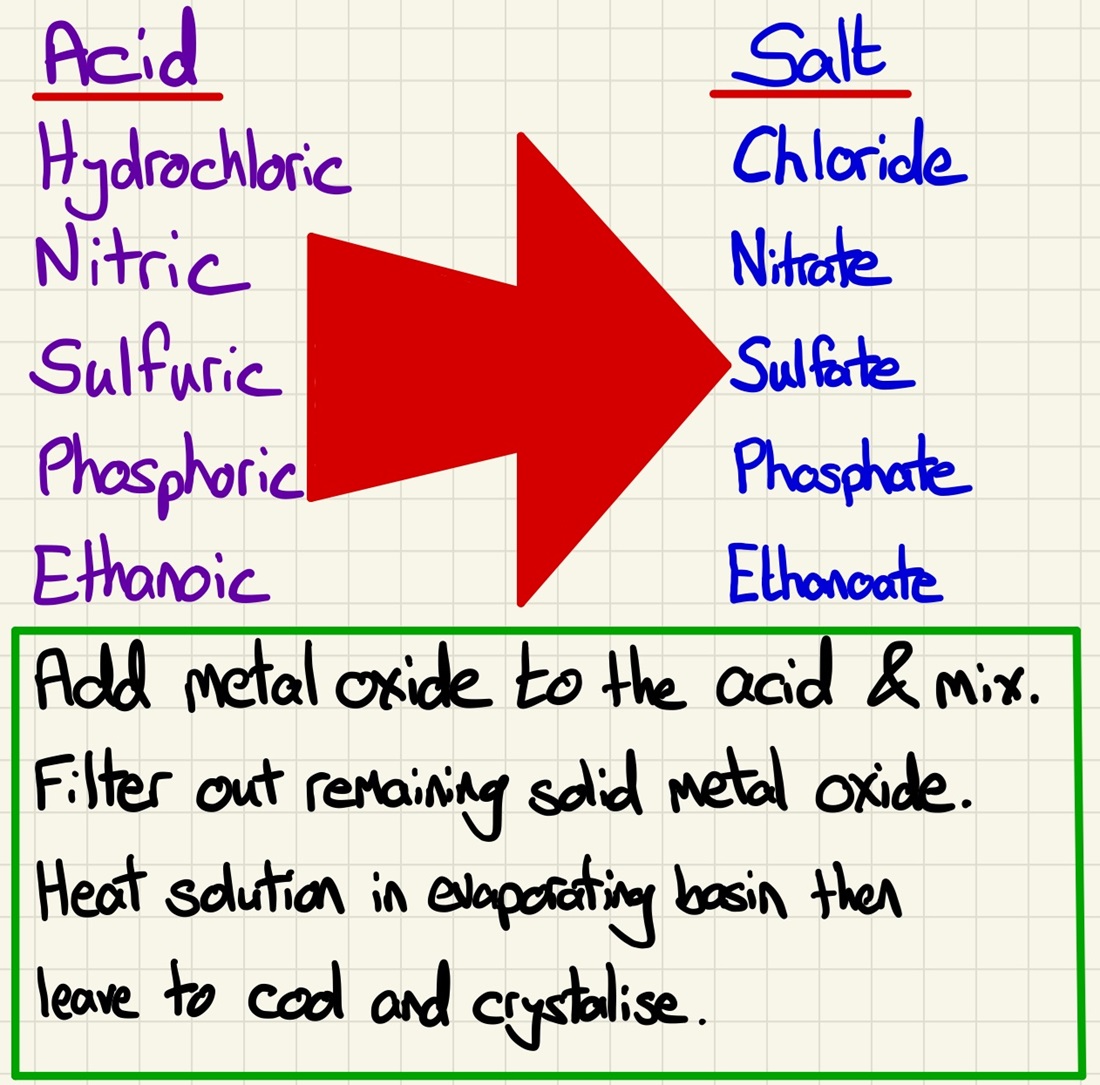
This brings together the standard equations that we have learned to a practical skill all about making a soluble salt. We firstly need to figure out which reagents we need to make a specific salt (take safety into account, if you want a sodium salt, you will never react sodium metal with an acid, always use dilute sodium hydroxide). Once we have decided on the reagents and have the balanced symbol equations, we can complete the required practical.
Strong and weak acids:
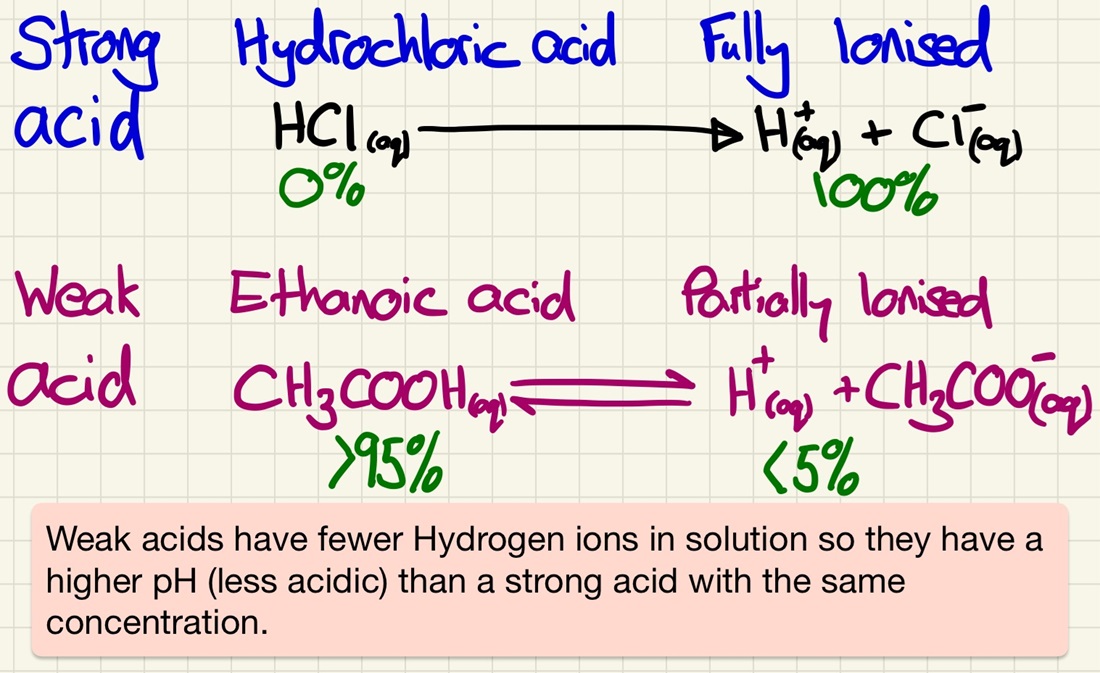
We must look at the differences between strong and weak acids. There is a very common misconception that strong acids are simply a more concentrated solution, however, there is a very distinct difference. Strong acids fully ionise or dissociate (release 100% of the H+ ions) and weak acids only partially dissociate/ionise (release less than 5% of the H+ ions).
Electrolysis of melts:
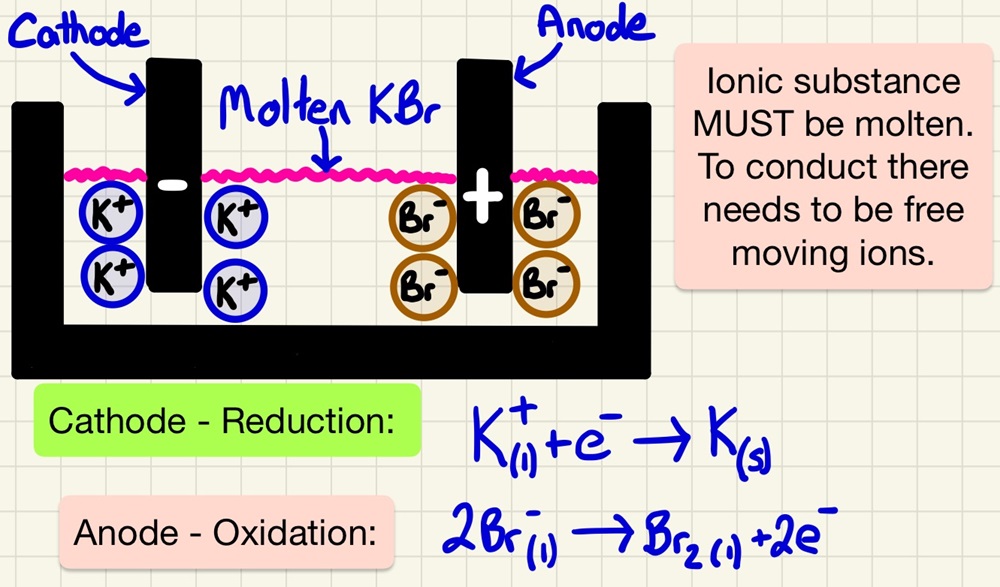
Electrolysis was discovered and made famous by Humphrey Davy and Michael Faraday who used it, among other things, to discover elements. We have already seen that unreactive metals (gold and platinum) are found as native or as elements in nature. Those metals less reactive than carbon can be reduced by it in a blast furnace. Metals more reactive than carbon are split from their ores using electrolysis. It simply splits compounds using electricity. In an chemical reaction to form an ionic compound, electrons are transferred (REDOX), in electrolysis, a current forces the electrons to return which splits up the compound into elements.
Electrolysis of Solutions:
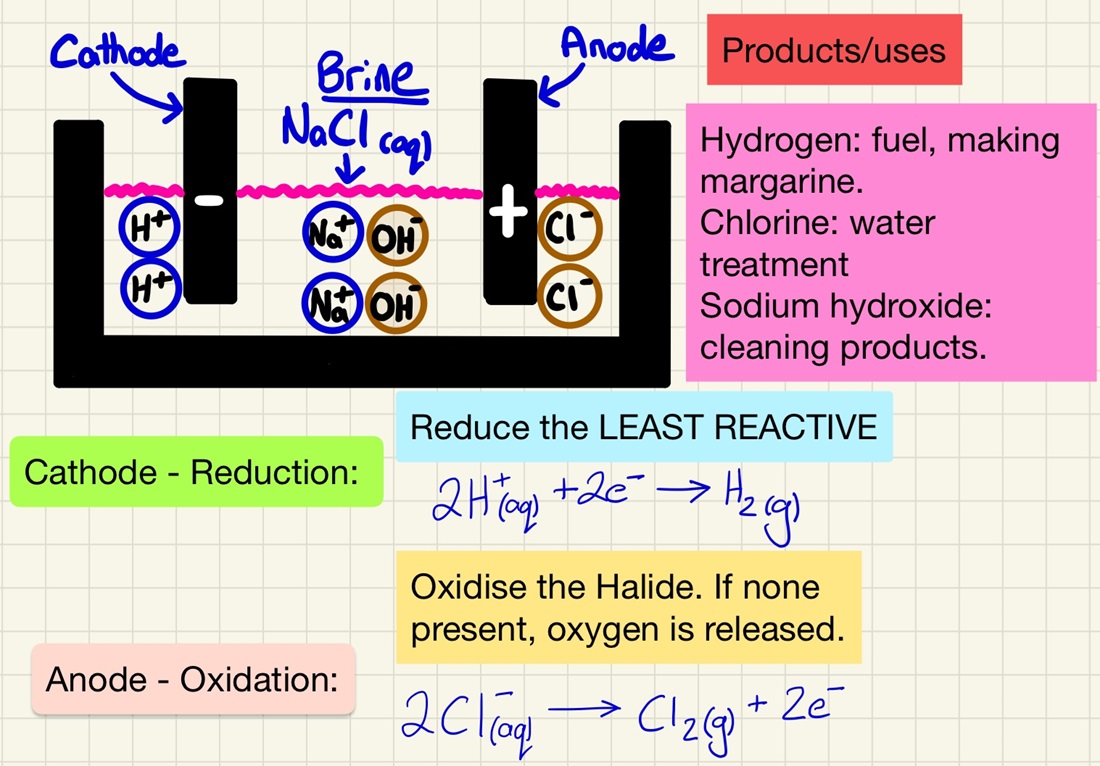
The same principles exist in this process, however, students often get confused about what is oxidised and reduced as there is a choice of 2 products. As it takes place in water, you need to be aware that there will be H+ and OH- ions present. Here are the rules:
Anode: If there is a group 7 ion in the solution, it will be oxidised and return to being an element, if not, oxygen will be produced.
Cathode: Whichever is least reactive out of the metal ion or hydrogen is reduced and forms on the cathode.
Remember the specific example of Brine. NaCl(aq) will produce Hydrogen at the cathode (used for fuels or making margarine), Chlorine at the anode (sterilising water or making bleach) and leave a solution sodium hydroxide (used in making cleaning products).
Key words/terms for this topic
Acid Alkali Anode Aqueous Base Bauxite Cathode Cryolite Displacement Dissociation Electrolysis Electrolyte Extraction Half equation Ion Ionic equation Native Neutralisation Ore Oxidation Reactivity Reactivity series REDOX Reduction Spectator ion State symbols
Curriculum Health Check:
Q: What is an electrolyte?
A: The solution containing the ions
B: The negative ions dissolved
C: The ions in the solution
D: The bubbles coming out of solution
What you need to know
Metals react with oxygen to produce metal oxides. This is the most basic example of oxidation. Loss of this oxygen is called reduction.

The reactivity series is an order of how vigorous metals react. We put hydrogen and carbon in as reference points to other reactions. More reactive metals can displace less reactive ones from a compound.

Reduction is a process of extracting metals from their ores (removing the oxygen). This is mostly the reduction using carbon such as in the blast furnace.

Metal + Acid → Salt + Hydrogen

Acid + Alkali → Salt + Water

Metal oxide + Acid → Salt + Water

Acid + Metal carbonate → Salt + Water + Carbon dioxide

During reactions with acids to make salts: hydrochloric acid makes chlorides, phosphoric acid makes phosphates, sulfuric acid makes sulfates and nitric acid makes nitrates. Using this, you should be able to predict the name of the salt being made and given a required salt, choose reactants that will make that salt.

Acids produce H+ ions and alkalis produce OH- ions. Their concentration links to the pH scale of 0-14 which can be measured using universal indicator or a pH probe. Neutralisation is shown by the half equation below:

H+(aq) + OH-(aq) → H2O(l)

Strong acids are fully dissociated of ionised. 100% of potential H+ ions are released into solution (nitric or sulfuric acids). In weak acids, only a tiny proportion are (ethanoic or citric acids). Don't mix dilute and concentrated with weak and strong.

Electrolysis is using a DC electric current to split a compound up into elements. A solution or molten substance undergoing this process is called an electrolyte. Positive ions move towards the negative electrode (cathode) and positive ions move towards the negative electrode (anode). There they change from ions to elements.

To remove elements from ores that are more reactive than carbon, we use electrolysis. Aluminium is the best example, it is melted and electrolysed. It is a very expensive process using lots of heat and electricity. Cryolite is added to lower the melting point of aluminium oxide (found in the ore bauxite). The electrodes are carbon (graphite).

When electrolysing a solution, the least reactive ions return to being elements. At the cathode (-) hydrogen is given off if the metal is more reactive than hydrogen. At the anode (+) oxygen is released unless there is a halogen in the solution, in which case it will be released (Cl2, Br2, I2). The oxygen and hydrogen come from the water itself. You should be able to predict what is going to be formed.

Extra topics needed for the Higher Tier papers:

Oxidation and reduction needs to be explained in terms of gaining and losing electrons. Relate reactions and their half equations to REDOX using OILRIG. Identify ions that are oxidised or reduced and identify spectator ions.

Describe the acid reaction with metals in terms of electrons (identify REDOX).

Write balanced ionic half equations for the reactions happening at both electrodes in all reactions. These include:

2H+ + 2e- → H2

4OH- → O2 + 2H2O + 4e-

4OH- + 4e- → O2 + 2H2O

This page was updated on: 16th February 2024


