Energy Changes
What we are learning:
Endothermic and Exothermic reactions:
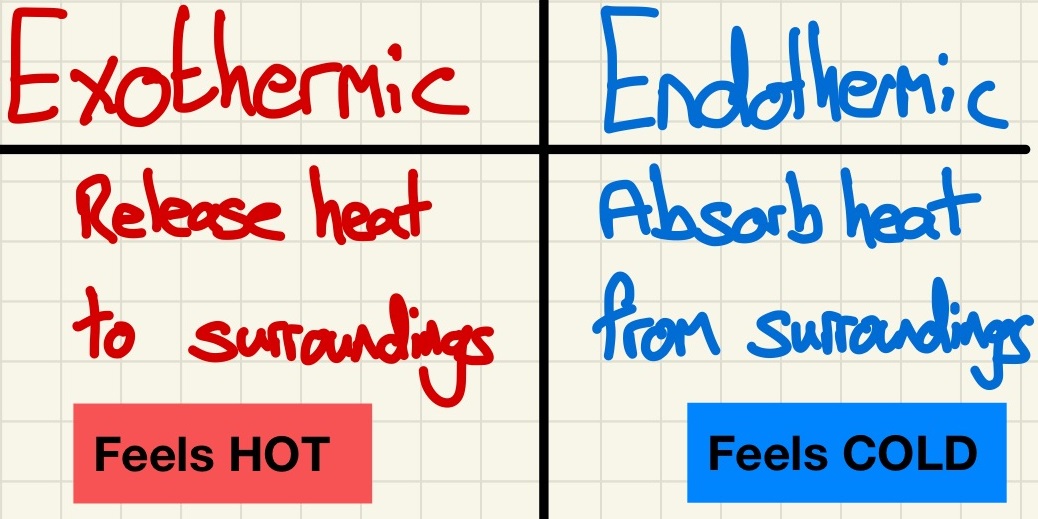
In a previous topic, we learned that mass is conserved, well, unsurprisingly, so is energy. The amount of energy in the universe is constant and it is transferred from one energy store to another. In this setting, it means that the energy at the start of a reaction is the same as the amount of energy at the end. If the environment of the reaction feels hotter, then there is now less energy stored in bonds, the surplus was released. This is an exothermic reaction, it feels hotter, energy is released. If there is more energy stored in the bonds of the products, this energy must have been taken from the environment so it will feel colder, this is an endothermic reaction.
Investigating temperature changes:

One of the required practicals that we do will investigate temperature changes. It is important to carefully record details such as: initial temperature, final temperature, mass of water, mass of reactant added, mass of burner/candle before and after. All of this information can be used to expand on our results. Firstly, it is easy to see that if it gets hotter - exothermic and if it gets colder - endothermic. To make this more challenging, we can involve our knowledge and use the equation from physics (specific heat capacity). This will allow us to calculate the temperature change per gram of reactant then the number of joules of energy released per gram of reactant and possibly even energy released in kJ/mol.
Reaction profiles:
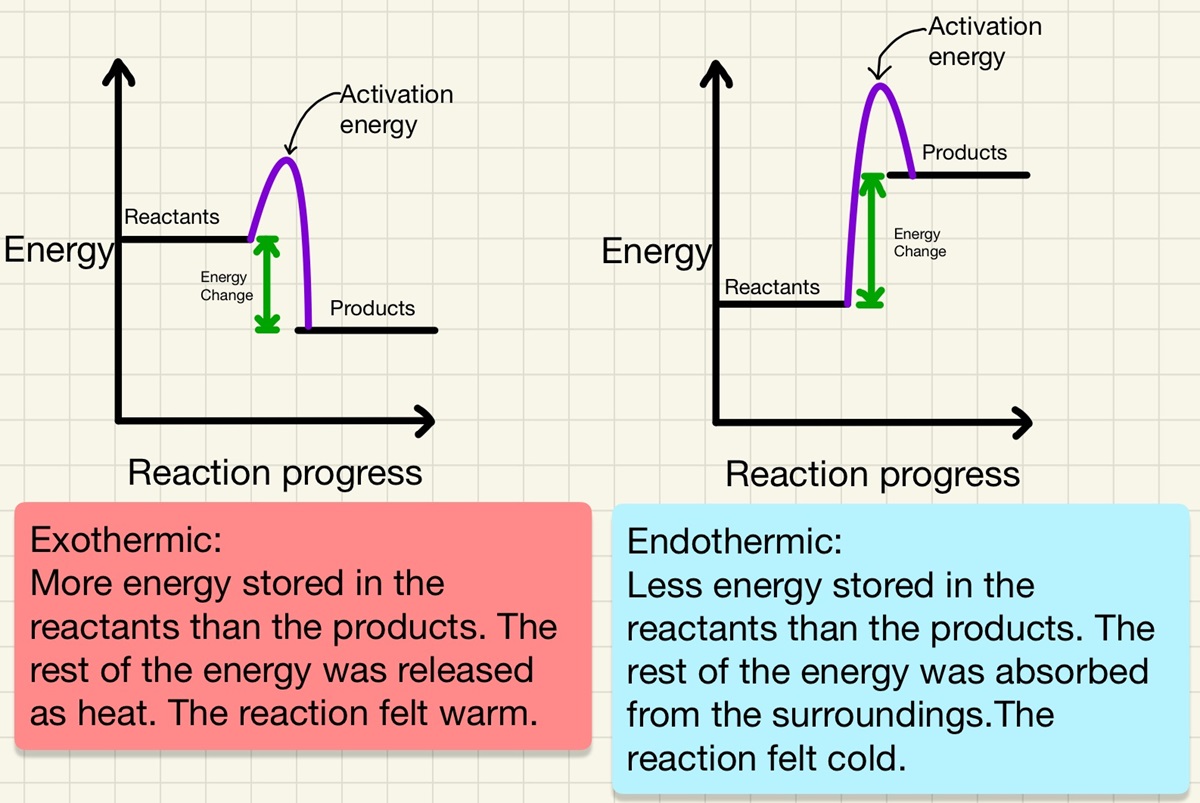
In a reaction profile, we can see the transfer of energy between the reactants and the products. Quite simply put, if there is more energy in the reactants (higher on the left) then it shows an exothermic reaction such as combustion, oxidation or neutralisation. If the reaction profile shows that there is more energy in the products (higher on the right) than the reactants, then it is an endothermic reaction such as thermal decomposition.
On the reaction profiles, there is a "hump" of energy between reactants and the products, this is the activation energy, the minimum amount of energy needed to start the reaction. We will learn more about this in the rate of reaction topic.
Bond energies:
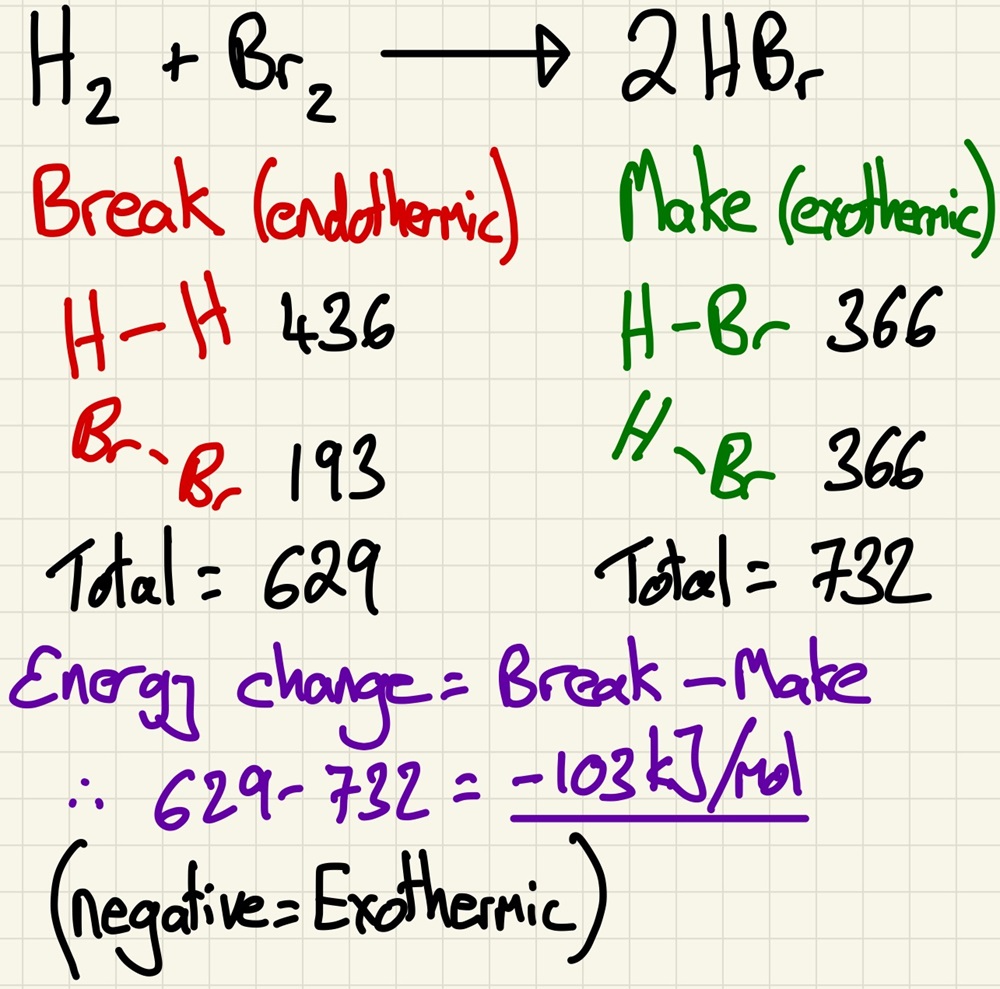
During a reaction, chemical bonds are broken then formed. The breaking of bonds is an endothermic process then the forming of new chemical bonds is an exothermic process. As we have the values of the mean bond energy for each type of bond, we can add up the total amount of energy needed to break all of the bonds in the reactants (on a balanced symbol reaction), we can then add up all of the energy that will be released when all of the bonds in the products are formed. The difference between these two numbers is equal to the amount of energy that is released (in an exothermic reaction) or absorbed (in an endothermic reaction).
Key words/terms for this topic
Activation Energy Bond Energy Combustion Endothermic Exothermic Neutralisation Oxidation Reaction Profile Specific Heat Capacity Thermal decomposition
Curriculum Health Check:
Q: The process of breaking bonds can be described as...
A: Exothermic for some bonds and endothermic for others
B: Exothermic
C: Energetically neutral
D: Endothermic
What you need to know
In a chemical reaction, energy is conserved. The energy before is equal to the amount of energy afterwards. Energy is stored in the bonds between atoms in a molecule.

Exothermic reactions release heat that was stored in bonds to the atmosphere (gets hotter). Combustion, oxidation, neutralisation. Used in handwarmers.

Endothermic reactions absorb heat from the surroundings and store it in bonds (gets colder). Thermal decomposition. Used in sports injury cold packs.

You need to be able to tell the difference based on experimental results and categorise exothermic or endothermic. You can then suggest uses for these reactions.

Required practical 10 - Investigate the variables affecting temperature changes in reactions like neutralisations.

You need to be able to draw reaction profiles for both endothermic and exothermic reactions. Ensure that you can label activation energy (minimum amount of energy needed to start a reaction off), reactants and products. Also, identify the overall energy change (difference in height between reactants and products.

Extra topics needed for the Higher Tier papers:

Bond enthalpy - in a reaction, energy is needed to break the bonds between the reactants. Energy is then released when bonds are formed making the products.

Given data about the energy required to break bonds (bond energy), you should be able to calculate the energy change in a reaction and say if it is exothermic (-) or endothermic (+).

This page was updated on: 1st June 2024


