Quantitative Chemistry
What we are learning:
Relative Formula Mass:
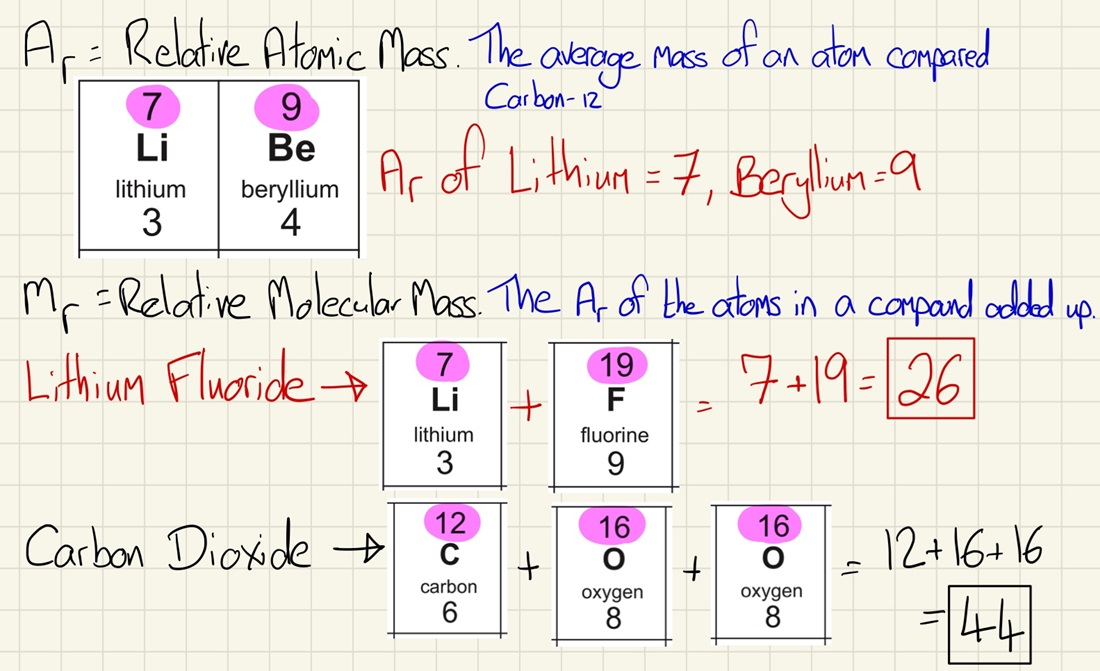
By using the relative atomic mass for an element, found on the periodic table (the bigger of the two numbers), we can find out the relative mass of an element. To find out the relative mass of a compound, you need to add up the relative masses of all of the elements within the compound and add them together.
In a balanced symbol equation, the total relative formula masses (Mr) of the reactants will be equal to the total formula masses of the products.
The Conservation of Mass & Percentage Mass:
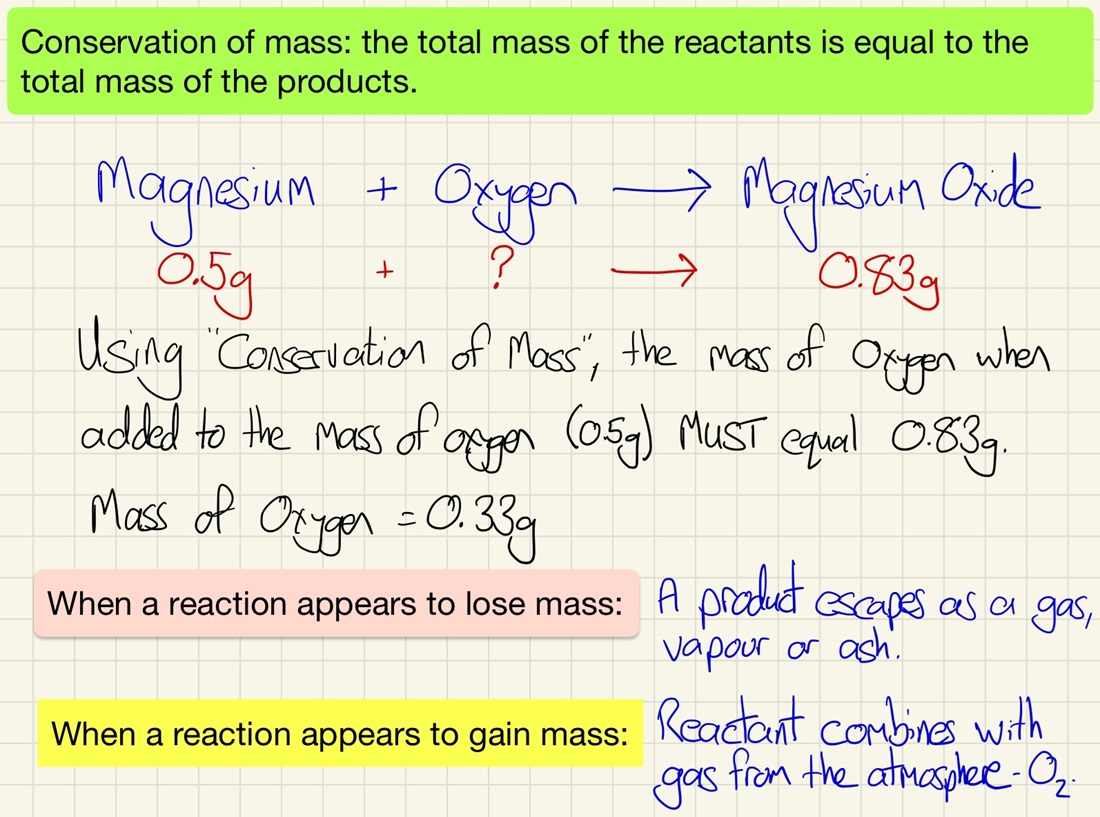
The law of conservation of mass is a very simple thing. Nothing ever disappears from a reaction and nothing "pops" into existence from nowhere by magic. Make sure that you can find out the mass of an unknown reactant or product using the masses of the rest. The mass going into a reaction (reactants) is always equal to the mass of the products.
The percentage mass is a very useful calculation. If you divide the Ar of an element by the Mr of the compound and multiply it by 100, you will find what percentage of the mass of that compound is because of that element. Once you have this value, you can work out how much of a metal is in an ore. If we work this out for Al2O3
27+27 / (27+27+16+16+16) x 100 = 53 %
We can now see that 53% of bauxite is aluminium, so if we have 200 kg of bauxite, we will be able to extract 106 kg of aluminium.
Moles:
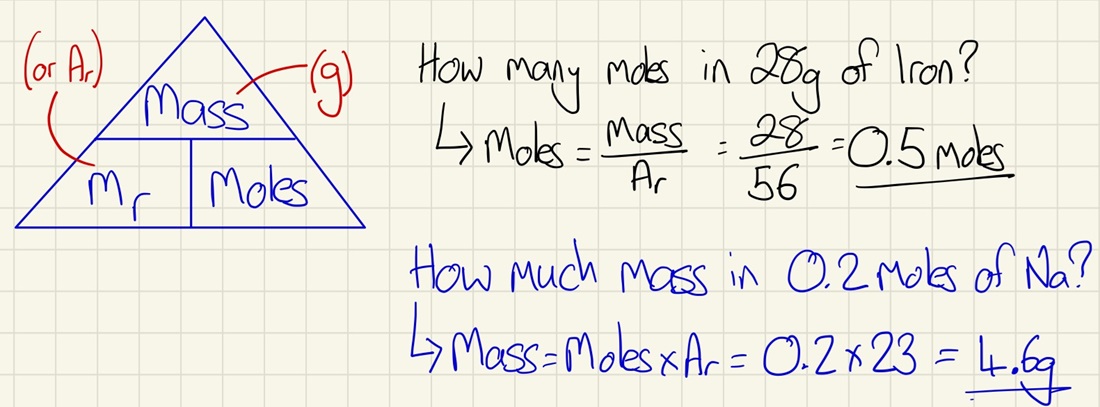
Don't be frightened! Just use the triangle in the picture and you will be able to work out the number of moles in a substance and also use the number of moles to calculate the mass.
Limiting reagents: In a reaction, if you add a specified mass of 2 reactants, one of them may be in excess, this means that the other is the limiting reactant (the one that will run out first). Once you have the masses, calculate the number of moles of each reactant you can see which one has more moles (if the balanced symbol equation shows them in a ratio of 1:1). If there are twice as many moles of one in the equations, there needs to be twice as many moles present.
More practice using moles:
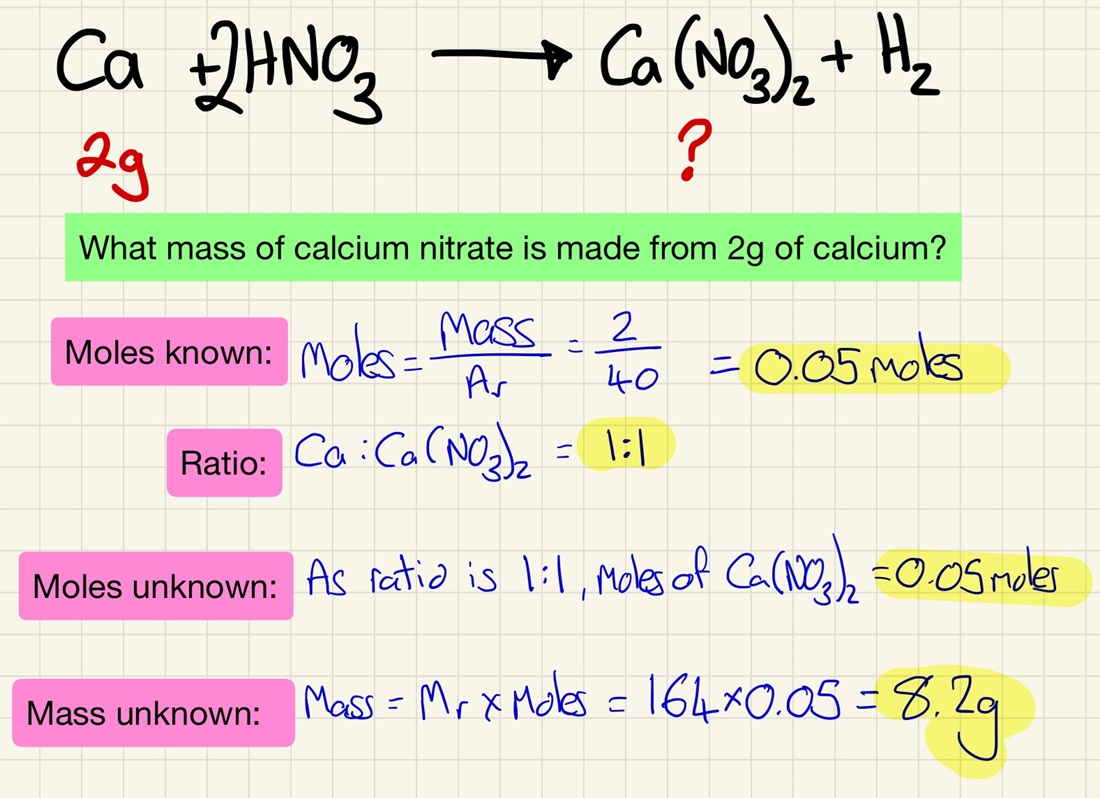
Learn the steps that I have in my model answer and use these every time that you complete one of these calculations.
Practice makes perfect!
Solutions:
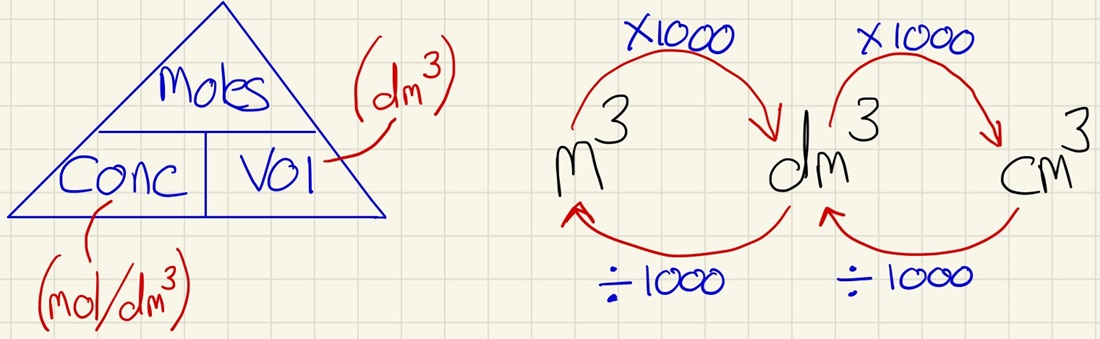
Solutions can have their concentration measured in 2 ways, either in g/dm3 or by using the extra step and converting the mass of the solute into moles and measure it in mol/dm3. Once you have the concentration, you can do further work and find unknown concentrations using titrations.
Titrations:
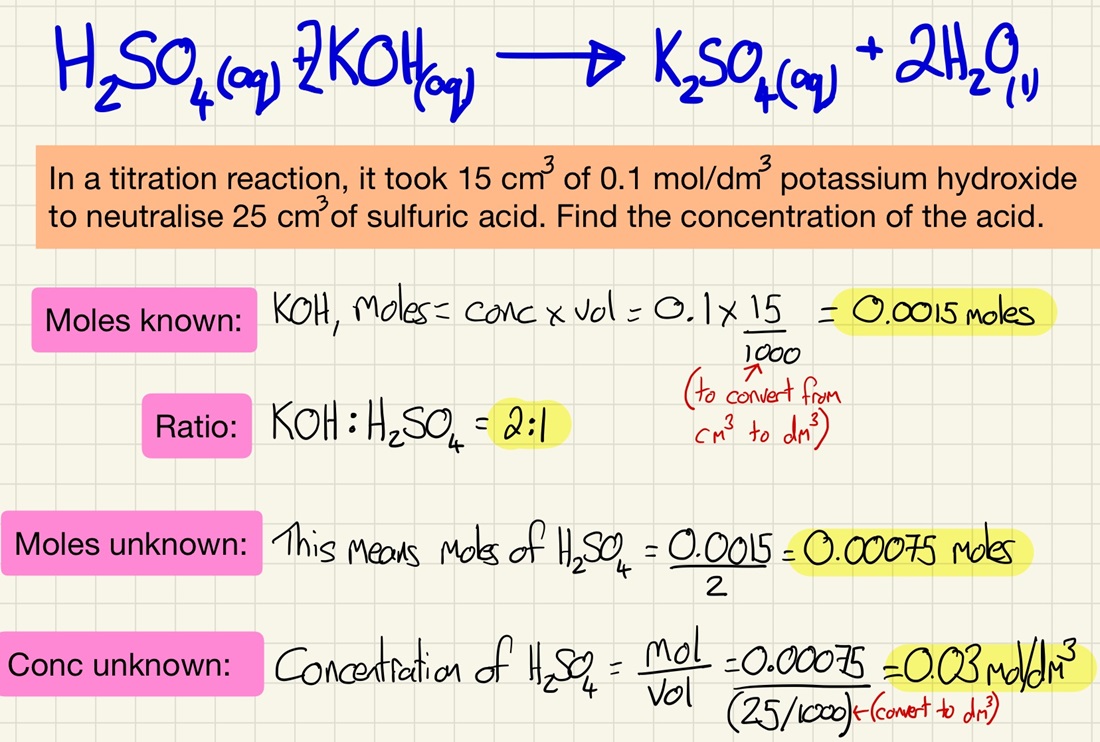
Here is the most challenging, and so the most rewarding part of quantitative chemistry. By reacting an acid of known concentration with an alkali of unknown concentration you can find out its concentration using mole calculations. A titration is an accurate way to measure a precise end point of a neutralisation. Follow the steps in my worked example and practice them over and over again.
Key words/terms for this topic
Avogadro's constant Concentration Conservation of Mass Dilution Excess Formula Isotope Limiting Reactants Mass Mole Neutralisation Reacting Masses Relative Atomic Mass (Ar) Relative Formula Mass (Mr)
Curriculum Health Check:
Q: Find the relative formula mass of FeCl3
A: 127
B: 162.5
C: 91.5
D: 163.5
What you need to know
The law of "conservation of mass" states that the total mass at the start of a reaction is the same as the mass after - not atoms are gained or lost.

By adding the Ar (relative atomic mass) of atoms in a compound, you can work out the Mr (relative formula mass). You can calculate the percentage mass of an element in a compound and use this to calculate what mass of an element there is in a known mass of a substance.

When one or more of the products in a gas, the reaction may appear to lose mass, it doesn't, some of the atoms simply escape as a gas but if they were trapped, conservation of mass prevails. Likewise, burning metals appear to get heavier, this is simply gaseous oxygen combining with the metal to form a metal oxide. The mass does not "appear", it was always there in the atmosphere.

Depending on the measurements you take and the equipment you use, there is a level of uncertainty. You need to be able to describe the uncertainty and suggest ways of improving it.

When a solid is dissolved in water, you need to be able to calculate its concentration in g/dm3.

Extra topics needed for the Higher Tier papers:

You need to be able to find out the number of moles (mol) in a substance using its mass and relative formula mass. This is linked to the Avogadro constant of 6.02 x 1023. Link this to equations, e.g. 1 mole of sulfuric acid reacts with 2 moles of sodium hydroxide.

Using balanced symbol equations, calculate the mass of a product or reactant using the mass of one that is known (reacting masses). Need to be able to apply ratios here too.

The limiting reactant is the one to "run out" first. You need to identify which one this is (other reactants are in excess) and use it in your calculations.

As well as the solutions noted in the foundation topics above, you need to be able to measure and calculate concentration in mol/dm3.

This page was updated on: 8th March 2024

