A2 - Acids, Bases and Buffers
Key learning for this topic
According to the theory of Brønsted-Lowry, an acid is a "proton donor" and a base is a "proton acceptor". You also need to remember the work that you completed on equilibrium as acid-base reactions are equilibrium reactions, especially when we move to weak acids and bases.

You need to calculate the pH of strong acids when given their concentration. You must also be able to calculate their concentration from their pH.
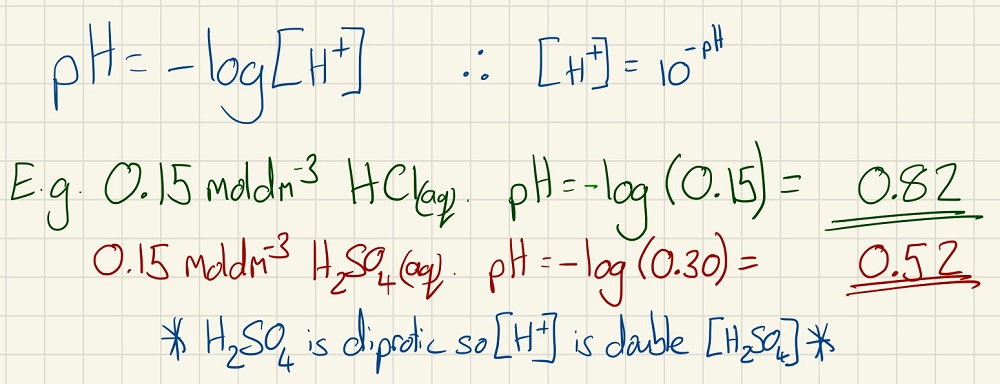
Before we move one alkalis, you need to understand that water is in equilibrium with its ionic products. If you are given the concentration of hydroxide ions, you can place that number into this equation and find the concentration of hydrogen ions which is needed to find the pH.
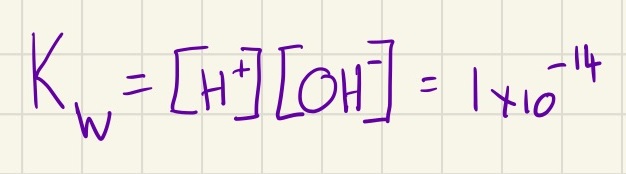
When calculating the pH of a strong alkali, combine the two processes above:
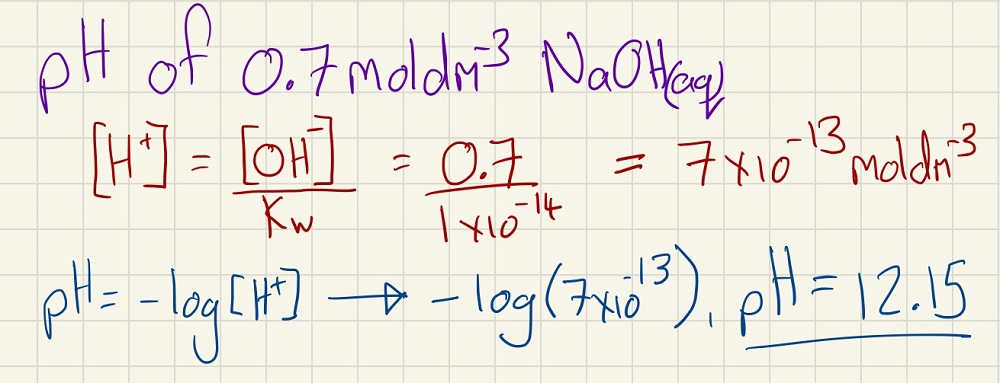
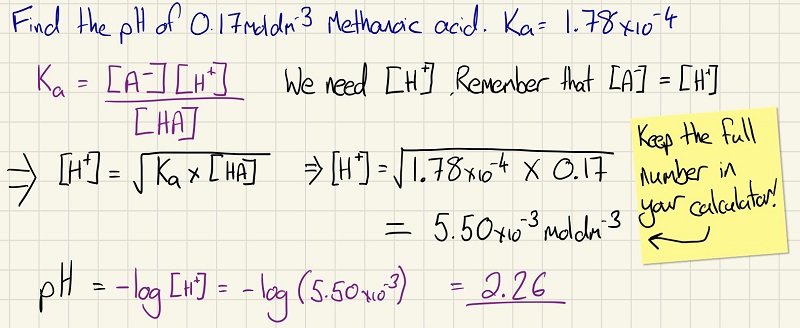
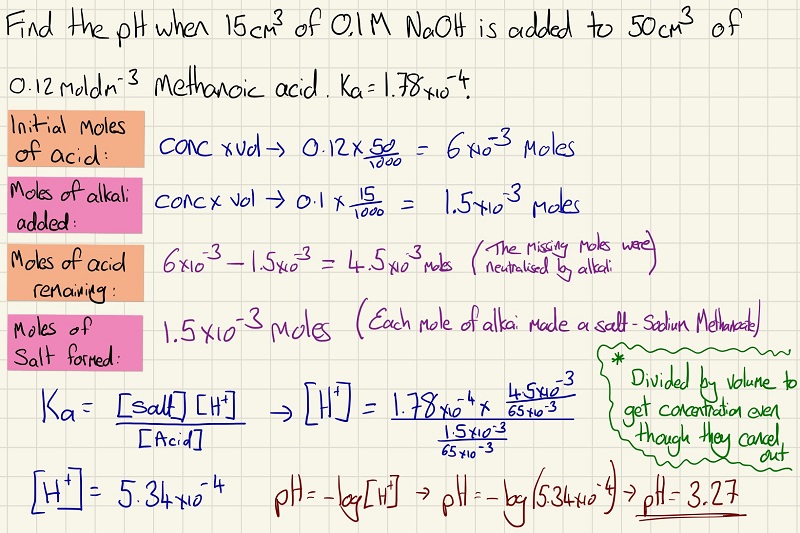
When finding the pH of a weak acid, you need to find the equilibrium constant of the acid as it will not be fully ionised or dissociated. See the calculation below to find the pH of a weak acid
Now we begin to see the neutralisation of a weak acid with a strong base. Firstly, we need to cancel out each mole of acid that has been neutralised by the base. In the equilibrium equation, however, there is another change. Rather than [A
If we add a small amount of acid to this buffer solution, we can calculate the new pH. The number of moles of acid added are added to the total moles of acid and removed from the total moles of salt.

Now if we return back to the original buffer solution and add a small amount of alkali, we can calculate the new pH. When we add alkali, the number of moles of alkali is removed from the total moles of acid and added to the total moles of salt.

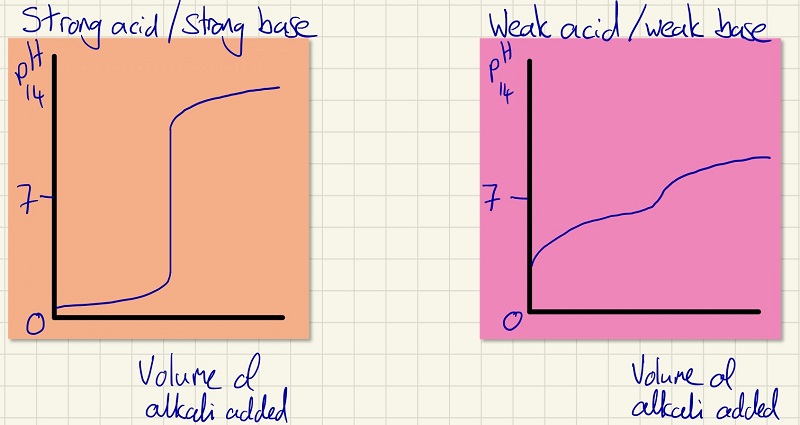
If we measure the pH often enough and at small enough intervals during a neutralisation and beyond, we can plot the curves. Below are two simple sketches. Remember the shapes, how suddenly strong acids/bases change and how there is more gradient for the weak ones. Note how much bigger and well defined the equivalence point is for strong ones too compared with the weak ones.
When selecting an indicator, remember that the colour change must happen after the equivalence point has been reached or the colour change may happen before neutralisation has happened.

This page was updated on: 2nd November 2023
