AS - Amount of Substance
Key learning for this topic
I adore this topic and there is no way to avoid it. My advice is to practice as many questions as you can and they will become second nature to you.

You cannot beat the basics: MOLES. You need to be able to work out the number of moles in a solid and in solutions and these equations are so simple?and you have to remember them as they do not get given to you in exams.
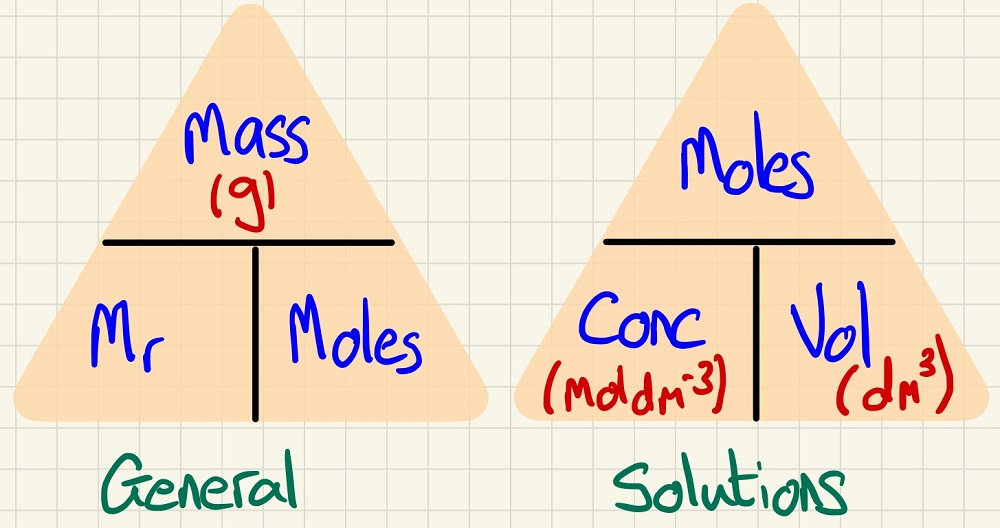
Make sure that you have a method for finding the mass of a product (for example) in a reaction:

1- Balance the equation

2- Find the number of moles of the substance given

3- Find the ratio (known : unknown)

4- Find the number of moles of the unknown

5- Convert those moles into a mass

Being confident with this and empirical formulae, yield and atom economy will prepare you for things that you will not have seen at GCSE such as the Water of Crystallisation. Remember that when drying out a crystal, you heat it to "constant mass", then you know that there is no more water to come out.
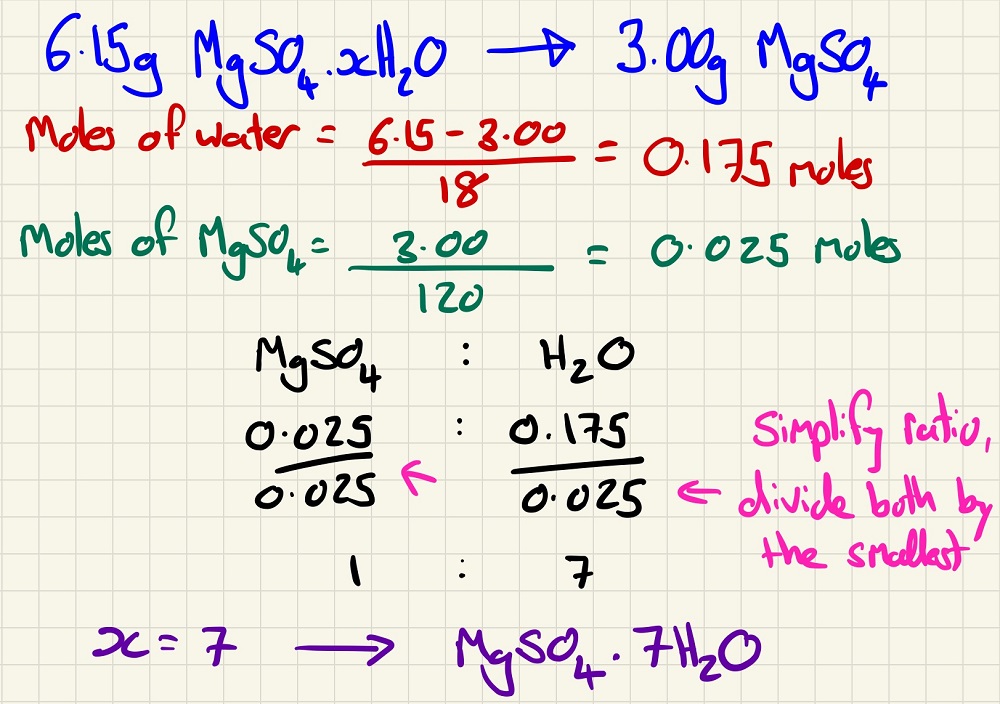
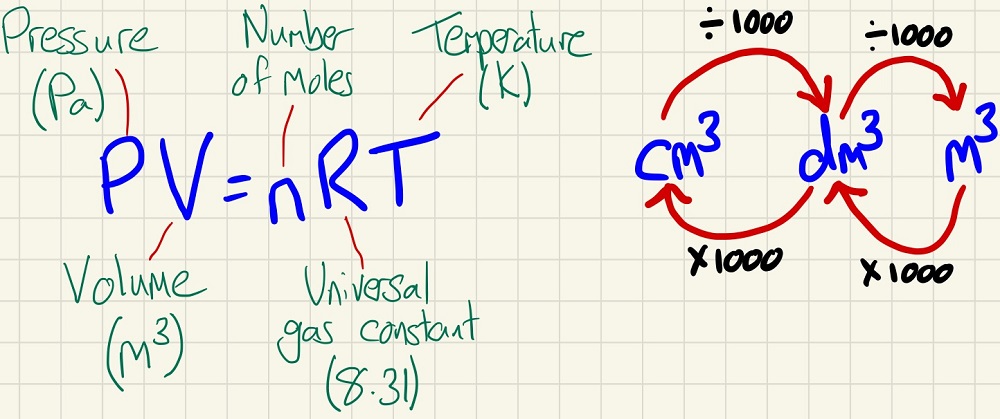
The last new thing that you will learn is the relationship between the number of moles of an ideal gas and its volume at a variety of temperatures and pressures. Please take great care with these units.
Finally, use all of the above together and you may start with a solid that dissolves, reacts then forms a gas. You've got this!

This page was updated on: 1st November 2023
