A2 - Aqueous Ions
Key learning for this topic
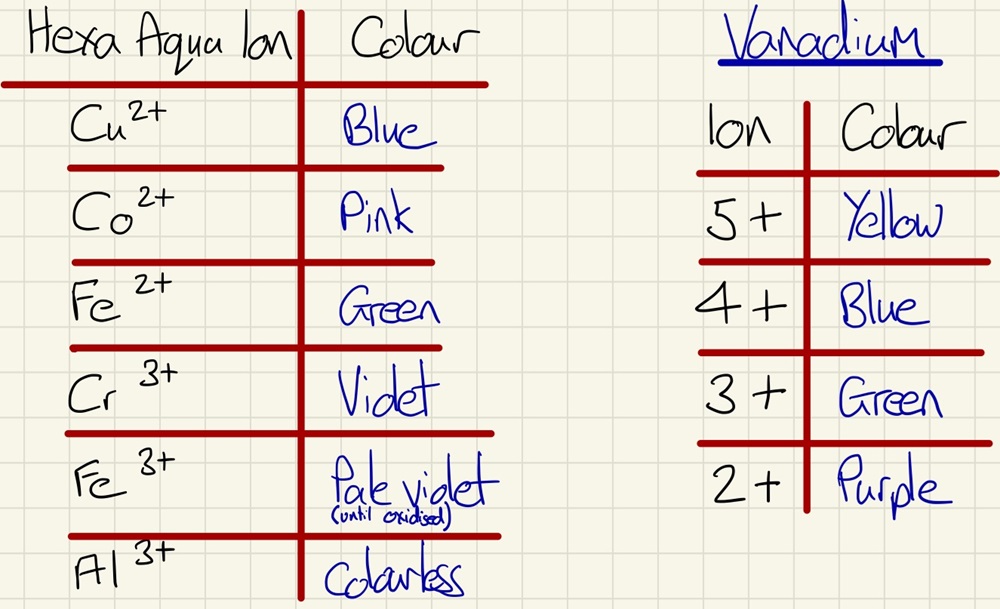
Depending on many factors including the ligands and the oxidation number of the metal, different complexes have different colours.
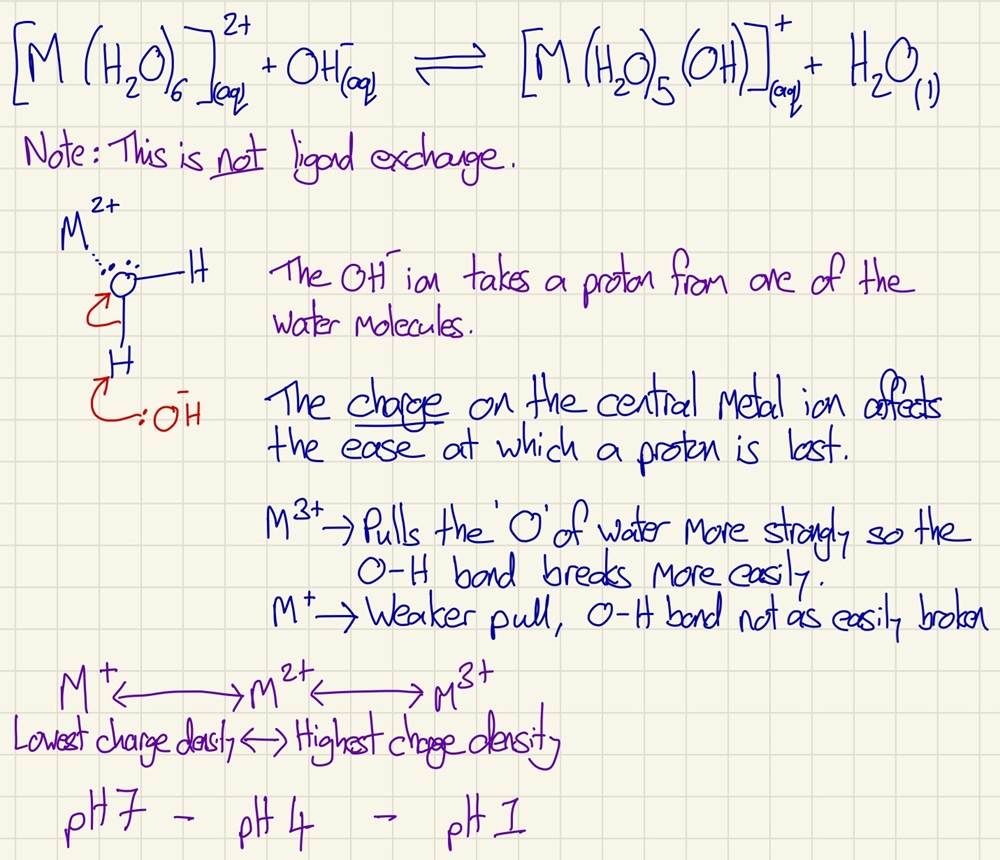
There are 3 main types of reactions that can occur here, they are Acid-Base (also known as hydrolysis), Redox (involving a change in oxidation state of the central metal in the complex) and Ligand exchange reactions.

During an acid-base reaction, a proton is taken from one of the water ligands. It is very important that you note that this is not a substitution reaction/ligand exchange.
The second type will have the oxidation state of the central metal change. For example, Vanadium is yellow in the +5 oxidation state, blue in the +4, green in the +3 and purple in the +2. These changes can be seen when a solution of vanadate ions are shaken with zinc, the solution will transition from yellow through to purple.

Finally, we will look at ligand exchange. These can change the coordination number which will affect the energy level change of falling electrons. This in turn affects the frequency of photons and so the colour that we see.

During an acid base reactions, a complex may lose a proton from one water molecule followed by one lost from a second and so on. As soon as the overall change of the complex reaches zero, the complex will precipitate out of solution. In certain cases, the complex will dissolve again if further acid-base reactions give it a positive or negative charge.
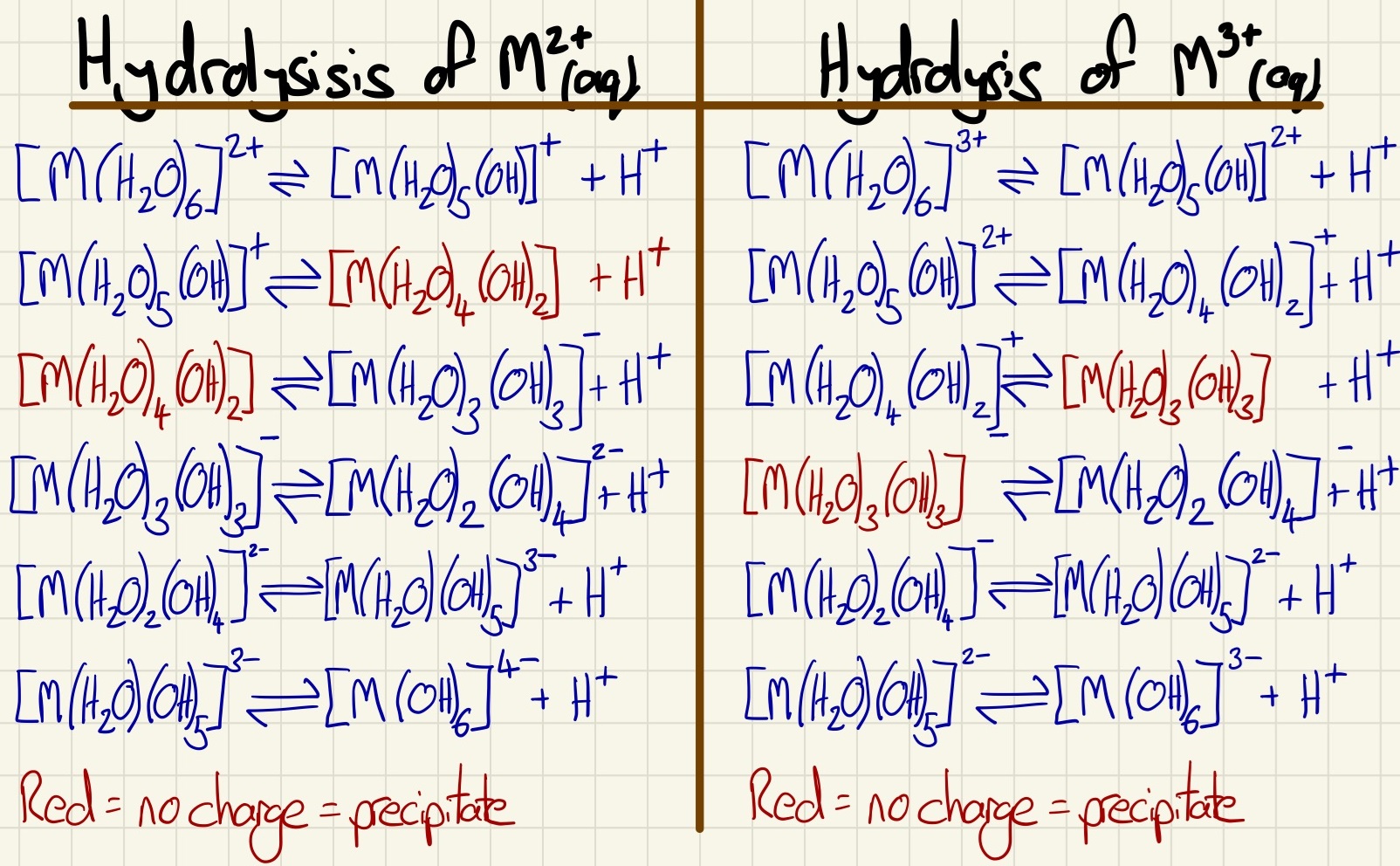
Ensure that you learn these reactions and that you can explain them in terms of the 3 main reaction types above.
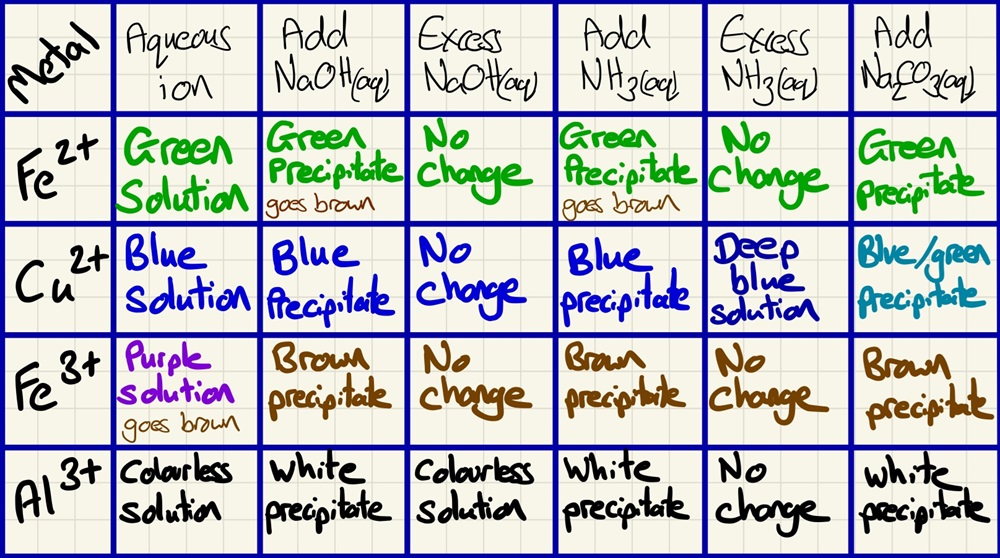
Although Aluminium is not a transition element and will not form a coloured complex, you need to be aware that it is Amphoteric. This means that it will behave like a base in the presence of acids (and react with them) and it will behave like an acid in the presence of bases (and react with them too).

This page was updated on: 7th November 2023
