A2 - Aromatic, Amines & Polymers
Key learning for this topic
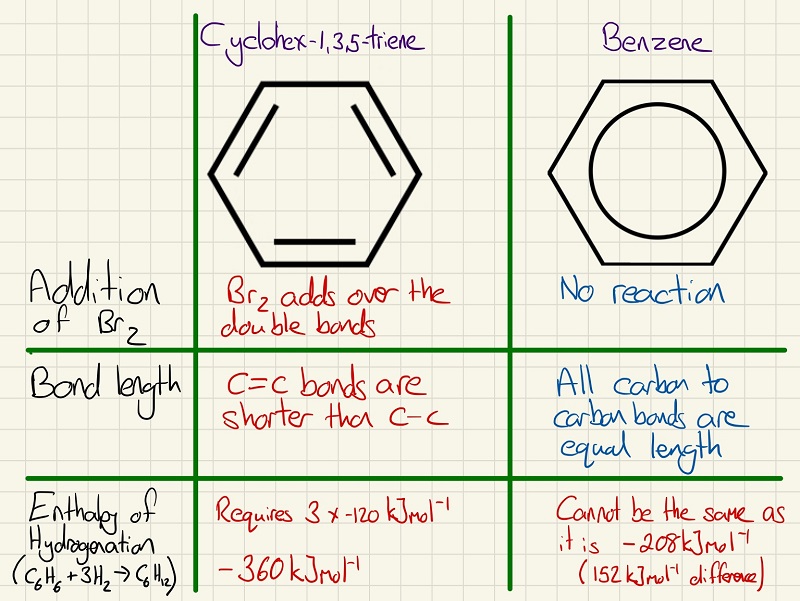
The structure of benzene was a mystery for some time and below we see the idea of a cyclic tri-ene and the benzene structure that we know today. Explore the three main pieces of evidence that separate the two structures.
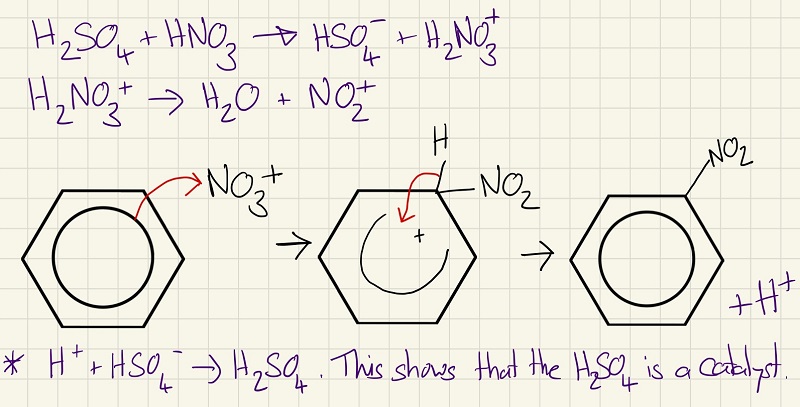
The reactions of benzene are very similar and you need to know how it reacts and the mechanisms. These are electrophilic substitution Firstly, the nitration of benzene.
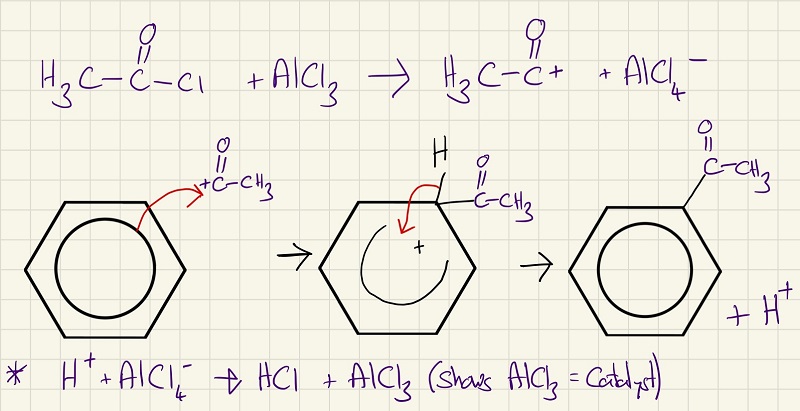
The second reaction is almost identical when you compare the mechanisms. This one is particularly special and it is called the Friedel-Crafts acylation reaction. This is particularly important as it adds carbons onto the benzene ring.
Amines are basic in nature, like ammonia, they can accept a proton from water and leave a hydroxide ion behind. As alkyl groups push electrons towards the nitrogen in an amine, a tertiary amine has a higher pH than a secondary amine, a secondary amine has a higher pH than a primary and a primary has a higher pH than ammonia. The odd one out is phenylamine. In phenylamine, the lone pair of electrons on the nitrogen are "absorbed" into the benzene ring so that they cannot attract a nearby proton. Quaternary ammonium salts do not have the word "amine" in their title as they are not amines due to the lack of the lone pair on the nitrogen. They have no basic character.

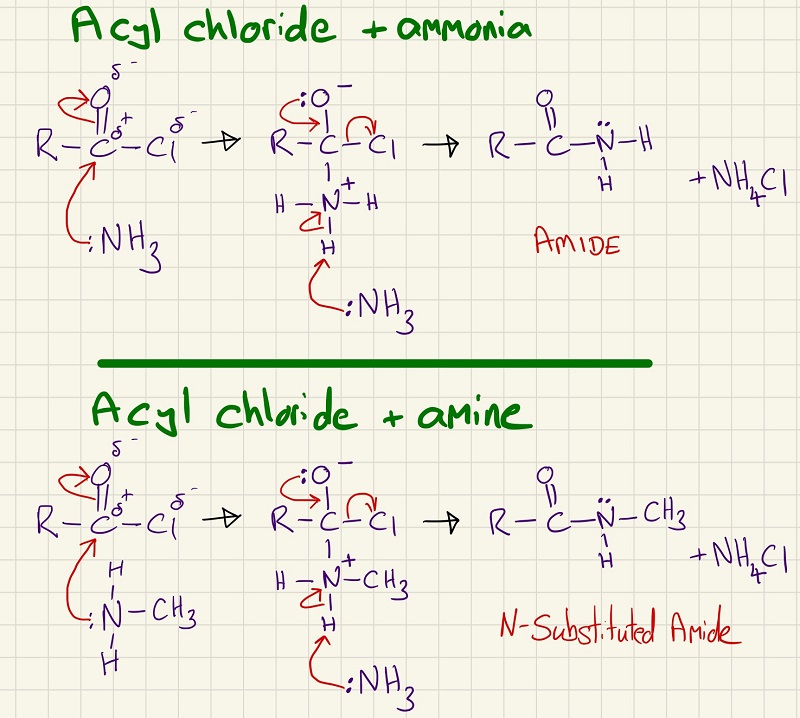
Amines are made by the nucleophilic substitution of a halogen alkane using ammonia. The two mechanisms that you need to learn are the reactions between an acyl chloride and ammonia (making an amide) and acyl chloride with an amine (making an N-substituted amide)
If you add an excess of ammonia to a halogen alkane, the reaction will finish with mainly quaternary ammonium salts. If you add an excess of the halogen alkane, you will mainly get the primary amine. This is still a rather messy way of making amines to order, the alternative is to react KCN with a halogenalkane (1 carbon shorter than needed as this reaction adds a carbon) then reduce the nitrile group into an amine. This way, you have more control about what is being made.

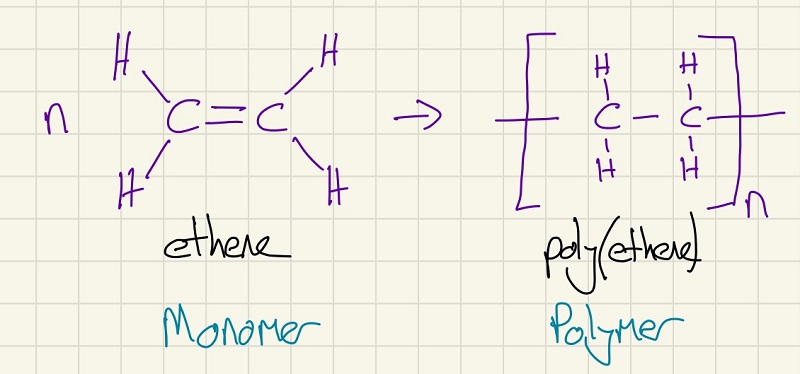
Additional polymerisation does not get much more complicated at A-level at all. Small molecules with a carbon-to-carbon double bond join to form a chain. Depending on the structure of the monomer (the number of carbons and the atoms attached to them), the polymer that is formed will have very different properties
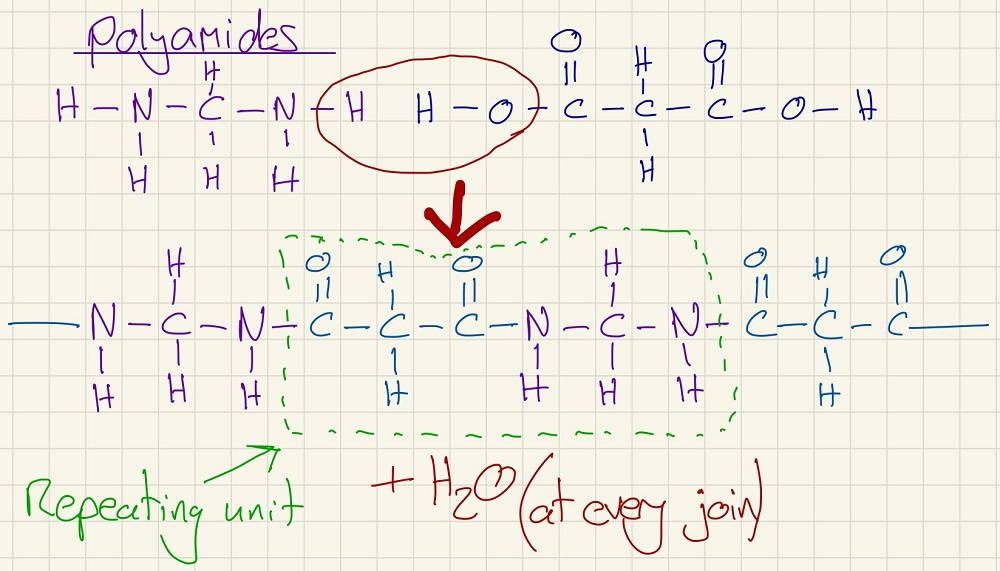
Polyamides are the first of two types of condensation polymers. When a condensation polymer is formed, a small molecule is released (this is very often water but is can be others such as HCl). In a polyamide, a dicarboxylic acid and a diamine polymerise, the name "polyamide" comes from the fact that each join is an amide bond. Examples include Nylon and Kevlar. The monomer in these is neither of the original molecules, instead it is one of each joined so that it has a carboxylic acid group at one end and an amine group at the other.
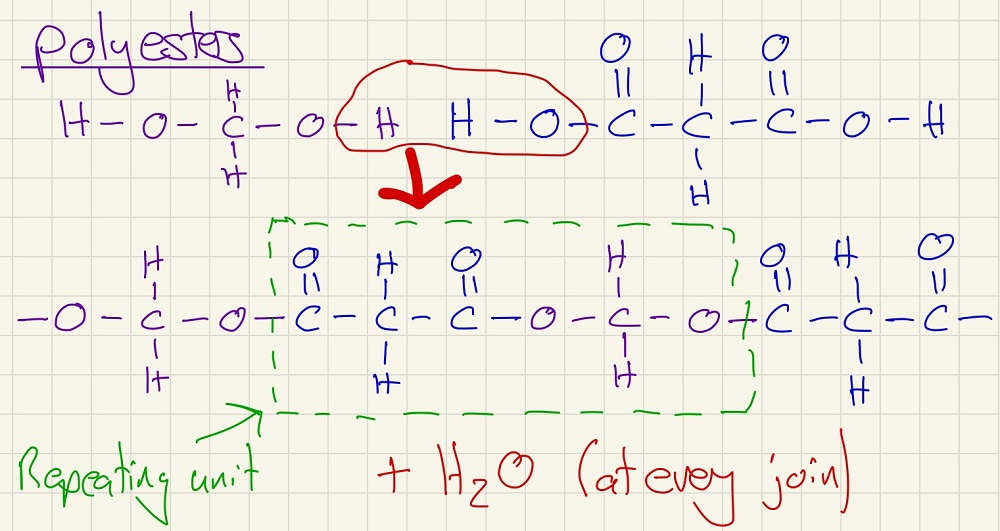
The second type of condensation polymer that we need to know about is the polyester. Like the polyamide, it gets its name from the fact that the linkage is an ester group. Here, we have diols and dicarboxylic acids joining and, again, the monomer is one diol joined to one dicarboxylic acid so that there is an alcohol group at one side and a carboxylic acid group at the other. Terylene is an example of a polyester, it is used as the synthetic fibres in clothes and bed linen.
Like with additional polymers, the properties of condensation polymers depend on the molecules that make them up. For example, Nylon 6,6 is a very specific form of Nylon with very specific properties. It is called this as the diamine has 6 carbons in its chain as does the dicarboxylic acid.

Finally, the strength of the polymer and other properties whilst depending upon the atoms on the chain, are due to the interactions those atoms have with those on the adjacent chains. These interactions may be Van der Waals' forces (all of them), but some may have permanent dipoles (such as the C=O on the polyesters) and even Hydrogen bonding (in polyamides, there are N-H bonds)

Addition polymers are chemically inert and if they go to landfill, they will not break down under bacterial action. Polyamides and polyesters can be broken down by bacteria. The natural reaction for this breakdown is hydrolysis, this is a natural reaction that occurs in nature. There is no natural reaction that breaks up the bonds in an addition polymer (because there are no polar bonds). If you want something stored for a very long time, use an addition polymer as polyesters and polyamides it will eventually break apart.

This page was updated on: 14th November 2023
