AS - Energetics
Key learning for this topic
This topic begins with a review of a GCSE idea that is covered in both Chemistry and Physics. Different substances require different amounts of energy to raise their temperature by 1K. This is the concept of Specific Heat Capacity. We almost always focus on water, by measuring the temperature change of a specified volume of water, we can calculate the amount of energy absorbed by the water and so (assuming 100% efficiency), the energy released or absorbed by a reaction.

Energy can be split up at A-level, we look at both components. Firstly (and most importantly) we have Enthalpy. Enthalpy is a measure of the energy change due to a change in chemical bonding. There is another component which has much less energy associated with it called Entropy. Entropy is a measure of the order/disorder of atoms. We will deal with it in more detail in Thermodynamics.

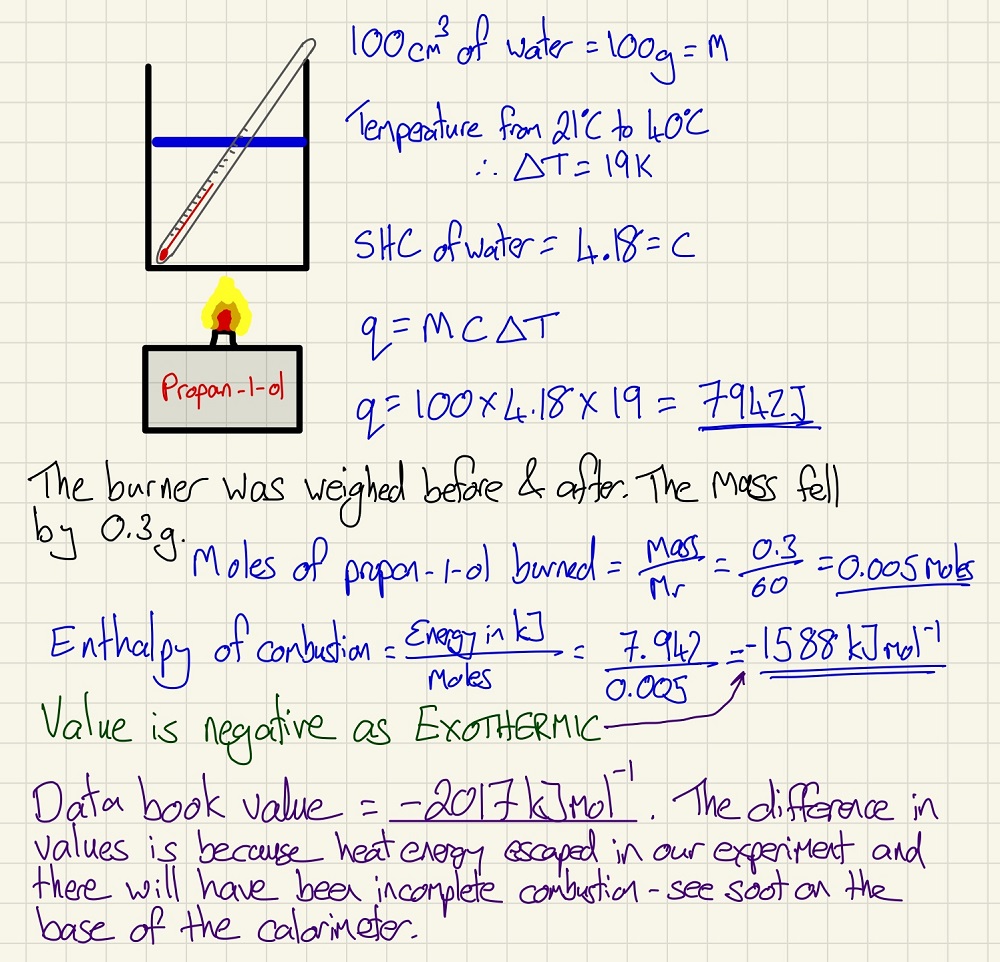
We measure these changes using a calorimeter, below is a basic version and a calculation that you are likely to have met. The values in data books for enthalpy changes are calculated using the most sophisticated equipment available, this is why newer publications are often slightly different in some values as the available apparatus is now better. You can use these calculation to find out the enthalpy of combustion of the fuel; find out the volume of water heated or even, if given the other variables, find the Mr of the fuel. There are so many uses for this.
Hess's Law states that the enthalpy change for a reaction is independent of the path taken. In simple terms, if you flew from Manchester to London, you would be 163 miles from where you started. If you flew from Manchester to Belfast, then to Cardiff and then to London, you would still be 163 miles from where you started.

We can use Hess's law to calculate the enthalpy change of a reaction without measuring it directly. In this example, we use the Enthalpy of formation (the enthalpy change when one mole of a substance is formed in its standard state from the constituent elements in their standard states). You can see that I have labelled the three "paths" A, B & C, taking great care with their direction, you can find the unknown path using the those that you can calculate.
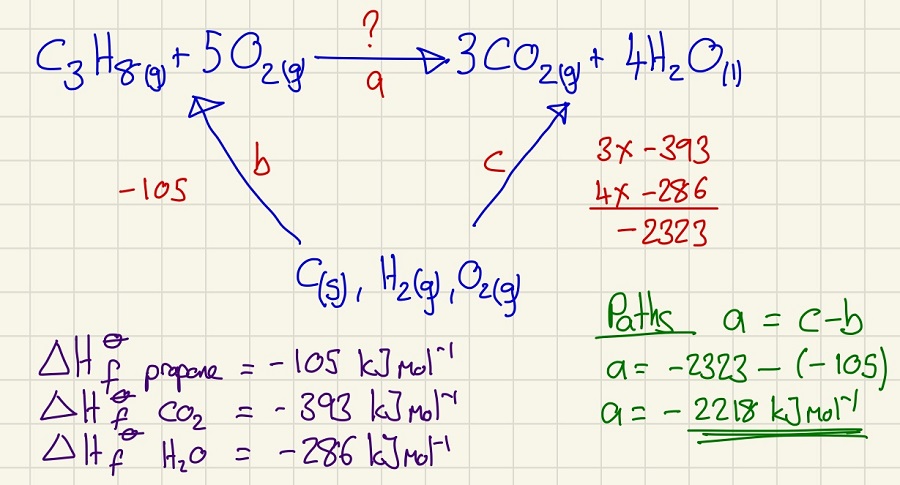
In another version, you may be given data for the enthalpy of combustion (the enthalpy change when one mole of a substance is completely burned in an excess of oxygen under standard conditions). These can also fit into the triangle, you just need to be careful that you understand what the data is that you are given. Many people have not spotted that the enthalpy of combustion of carbon is the same value and equation as the enthalpy of formation of carbon dioxide. The same goes for other oxides.

Finally, we can look at bond enthalpy. Breaking bonds in endothermic and making is exothermic, use this to help check that you have the arrows the right way around. This is first taught at GCSE so it should appear familiar. This method is rarely used in practice as these values are the MEAN bond enthalpy and as the environment in which the bond is can cause variances due to polarity, you will never get an exact match.
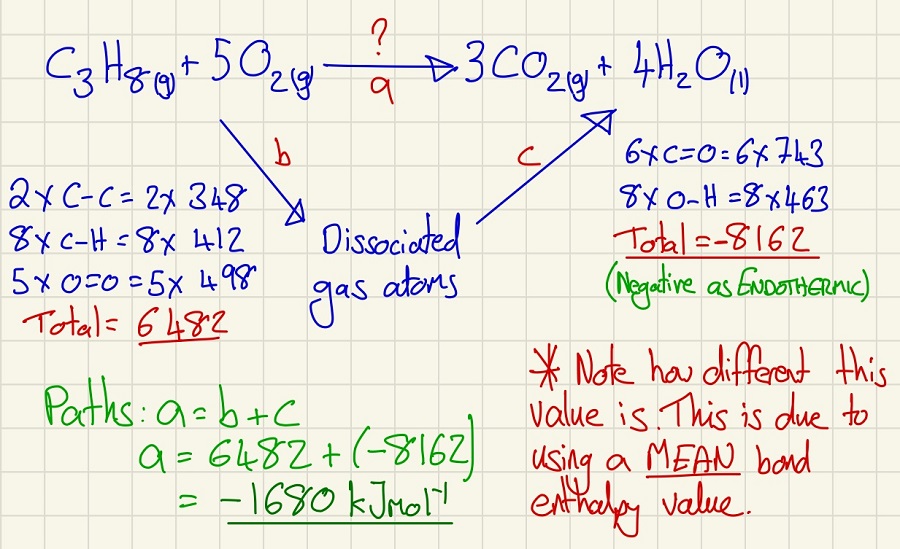
Same reaction that can have its enthalpy calculated 3 ways. Calorimetry, by enthalpy of formation and by bond enthalpy. Think about their pros and cons and link them to the required practical.

This page was updated on: 10th January 2024
