A2 - Equilibrium and Gases
Key learning for this topic
This topic builds directly onto the equilibrium topic. Here, we are looking solely at gases. Remember that one mole of any ideal gas will occupy the same volume at the same temperature.

Firstly, we need to find out the "mole fraction" of each gas in the equation. Both reactants and products.
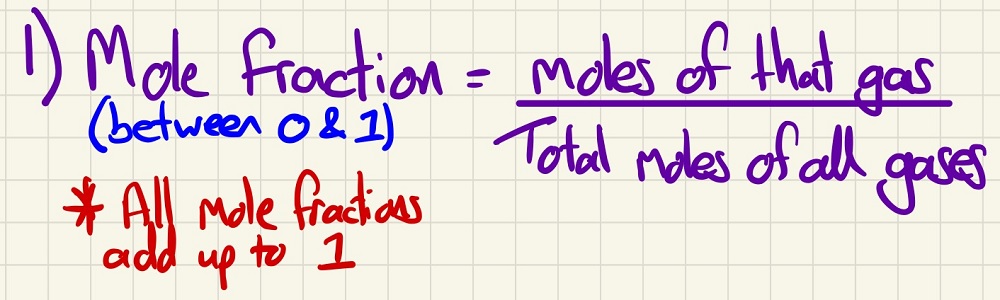
Next, we need to find the partial pressure that is exerted by each gas (reactants and products again).

Finally, put these partial pressures into the equation and use them to calculate Kp for the equilibrium at that particular temperature.
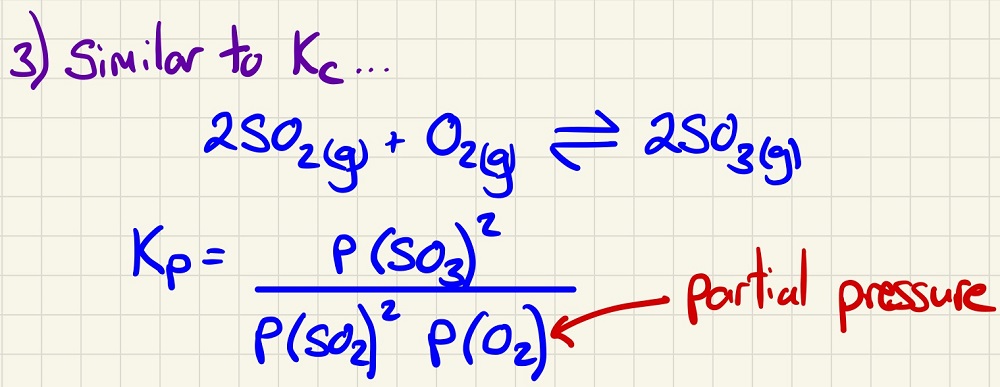
Alternatively, you may have some partial pressures and the value of Kp and need to find a missing partial pressure. This may then give you the total pressure in the system.

This page was updated on: 10th October 2022
