Organic Chemistry
What we are learning: (component knowledge)
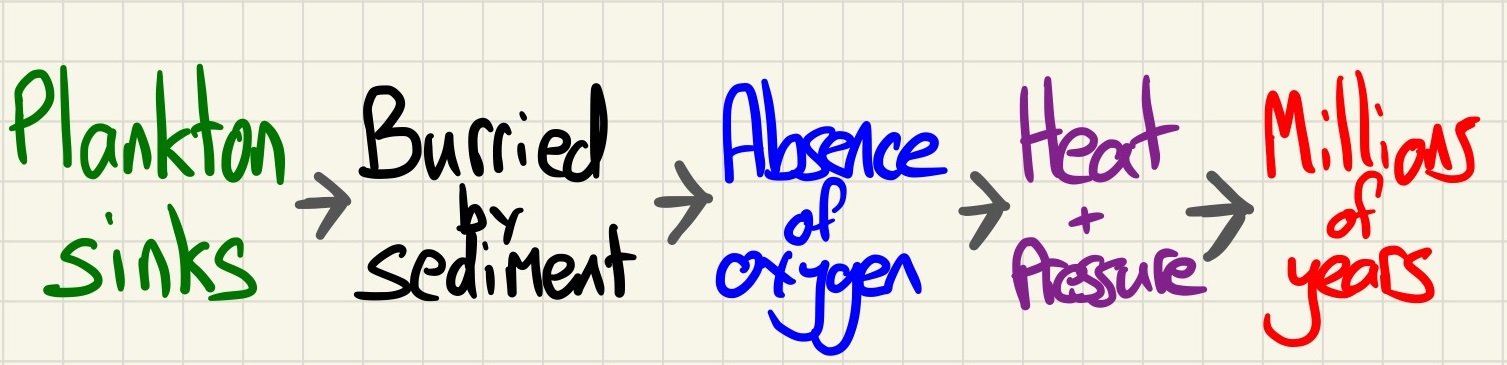
Formation of crude oil:
Crude oil is a finite resource. Although it is being formed right now, it takes a long time and we are using it far faster than it can be created. The remains of plankton and other ancient biomass sank to the bottom of the sea where it was covered in mud. It was then subjected to deeper burial which caused great pressure and heat over millions of years to transform it into crude oil. In this process, it is important to note that it was formed with a certain amount of luck. If not for the layers of mud forming an impermeable layer above it (cap rock), the gas and oil formed would have escaped. Likewise, if there was not an absence of oxygen at the initial burial, then the biomass would have simply decomposed.
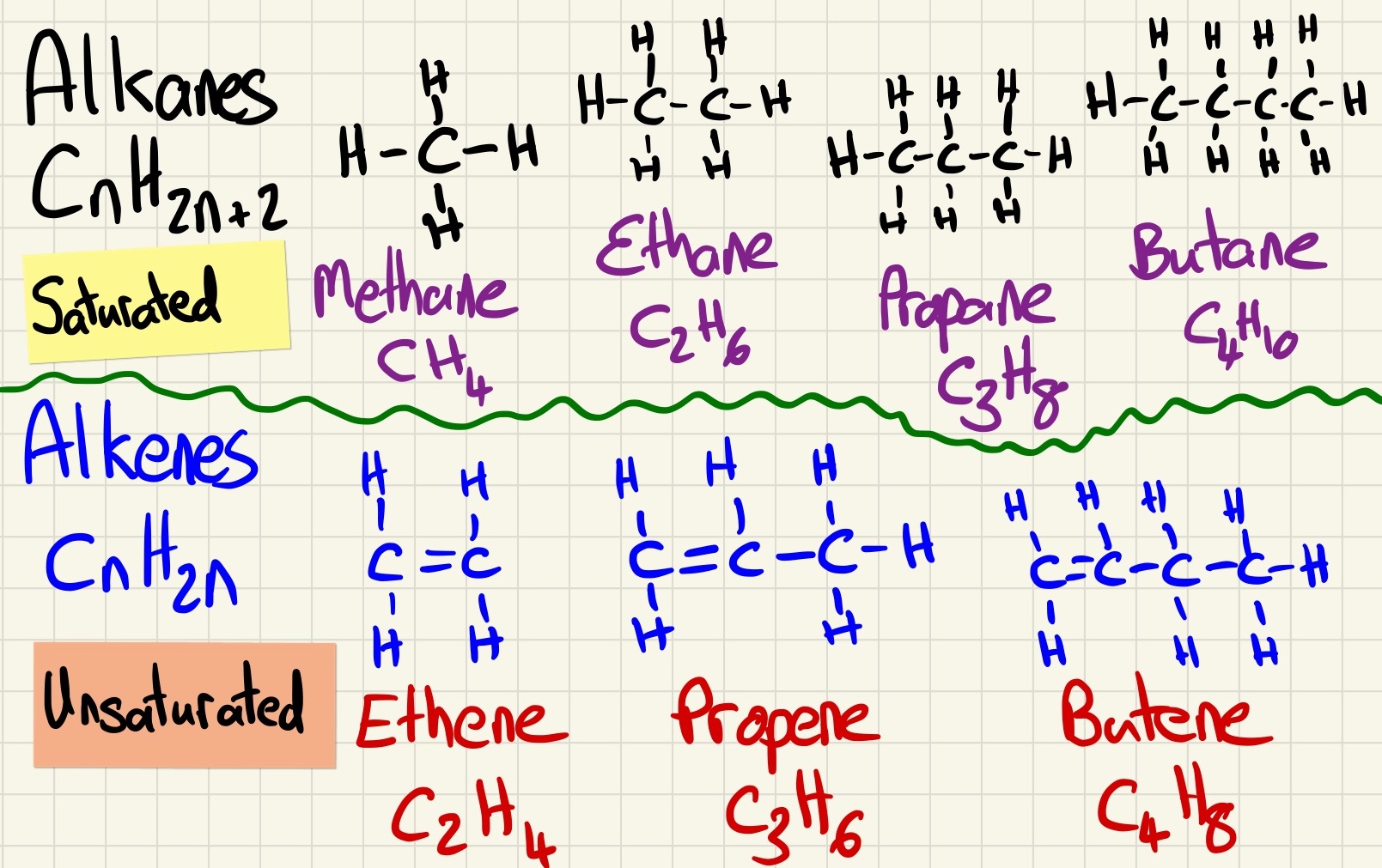
The crude oil is made up of a wide variety of hydrocarbons, compounds made up exclusively of hydrogen and carbon (no oxygen or there would be nothing except water and carbon dioxide left). The most important of these hydrocarbons are the Alkanes. These have a general formula that you can use to identify them, they all have this general formula:
CnH2n+2
The first 4 need to be remembered:
Methane CH4
Ethane C2H6
Propane C3H8
Butane C4H10
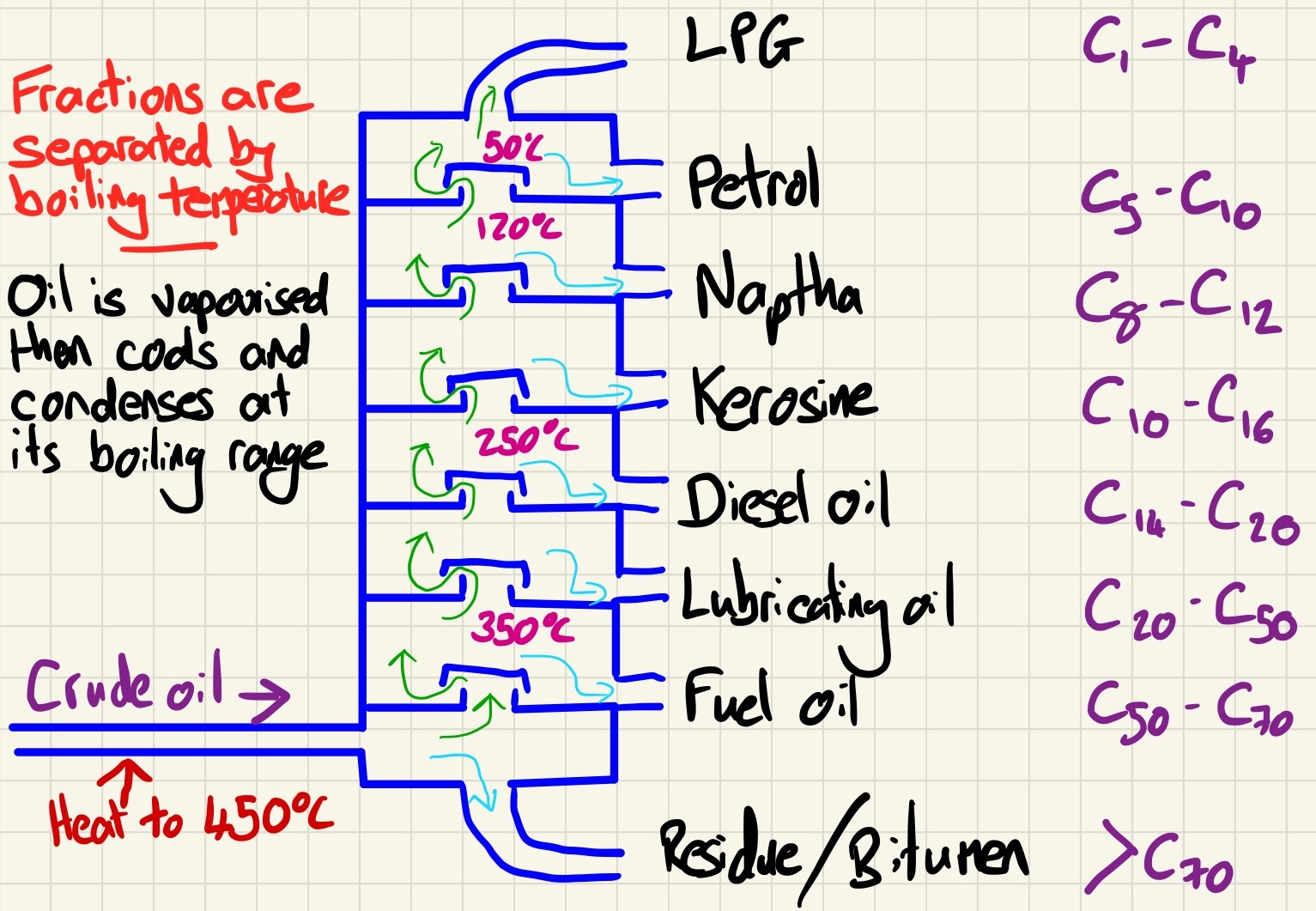
Fractional distillation:
Crude oil is a useless substance on its own. It must first be separated into different fractions. Remember that a fraction is not a single compound, instead, it is a collection of alkanes that have a similar boiling point and so a similar chain length. The process is fairly simple but you must remember to use the key vocabulary for each stage.
1. The crude oil is vaporised as it is heated to around 400°C.
2. It is fed into the fractionating column at the bottom so the vapours rise. The column has a temperature gradient (hottest at the base, coolest at the top).
3. Fractions condense back into a liquid at different heights depending on their boiling points.
4. Fractions with a high boiling point (long chain) will condense near the bottom.
5. Fractions with a low boiling point (short chain) will condense near to or at the top.
The fractions are collected for use as either fuels or feedstock. (Feedstock is nothing to do with food, it is simply a term to describe a chemical that is used to make something else).

The uses and properties of each fraction:
So far, we have met the relationship between length of chain and boiling temperature. The longer the alkane chain, the stronger the intermolecular forces so the higher the boiling temperature. Remember that methane is the shortest chain and it only has one carbon and it is a gas at room temperature and road tar (which has very long chains and is not even properly vaporised in fractional distillation) is a solid at room temperature. We also learn that short chains are volatile, easy to ignite, fluid and burn with a clear flame. Conversely, long chains are not volatile, difficult to ignite, viscous and burn with a smokey flame.
C1-C4 - LPG: Fuels for cooking and heating
C5-C10 - Petrol: Fuels for cars
C8-C12 - Naphtha: solvents and making other chemicals
C10-C16 - Kerosene: Aircraft fuel
C14-C20 - Diesel oil: Fuel for large vehicles
C20-C50 - Lubricating oil: Lubrication of machines/engines
C50-C70 - Fuel oil: Ship engines and heating oil
C70- and above - Residue/Bitumen: Tar for flat roofs and roads.
Cracking and the uses of alkenes:
The demand for certain chain lengths is higher than others. We need much more petrol than we need longer chains like fuel oil and residue. Cracking is a process in which the longer/lower demand fractions are heated with catalyst and they break down into smaller fractions and an Alkene. An Alkene is also a hydrocarbon, however, it contains a carbon to carbon double bond C=C. Alkenes have the general formula of CnH2n and they react with bromine water. This is the test for an alkene, they decolourise bromine water (orange to colourless).
Alkenes have another excellent property, under pressure and in the presence of a different catalyst they will join together to form a very long chain. The individual alkene is a monomer and the long chain is a polymer (plastic). Depending on the structure of the monomer, the polymer will have very different properties (rigid, flexible, transparent, non-stick etc.).
When hydrocarbons burn in plenty of oxygen, they undergo complete combustion which produces CO2 and H2O. If, however, there is not enough oxygen, there will be incomplete combustion in which there will be C (soot), CO (carbon monoxide) and H2O. The production of carbon monoxide is very dangerous as it can be lethal to people. Always have a carbon monoxide alarm in rooms where you have gas appliances or fires. Have gas appliances regularly serviced by a qualified professional and check that appliances in holiday homes etc have appropriate certificates.
Key words/terms for this topic
Alkane Alkene Boiling point Catalytic Cracking Combustion Complete Combustion Crude Oil Decolourising Feedstock Flammability Fluidity Fraction Fractional Distillation Fuel Homologous Series Hydrocarbon Incomplete Combustion Saturated Unsaturated Viscosity Viscous Volatile
Quick Quiz:
What you need to know
Where crude oil is found today, how it was formed and that it is a finite resource. You need to know that crude oil is a mixture of many different organic compounds and it needs to be separated into fractions to be useful. You need to know, describe and explain the process of fractional distillation and the names and uses of a selection of these fractions.

Alkanes and alkenes are examples of hydrocarbons, you need to know the names of the first 4 alkanes and show their formula. e.g. ethane is C2H6.

There are clear patterns between the length of the carbon chain and viscosity, flammability and boiling temperature which you need to know.

Catalytic cracking is a process in which long alkanes and be broken down into smaller alkanes and alkenes. The alkanes can be used in fuels whereas the alkenes are used to make polymers (plastics).

This page was updated on: 4th March 2024
