A2 - Rate Equations
Key learning for this topic
As always, ensure that you have revisited the last topic to closely link to this one which was AS Kinetics. Make sure that you are up to speed on what affects rates of reactions.

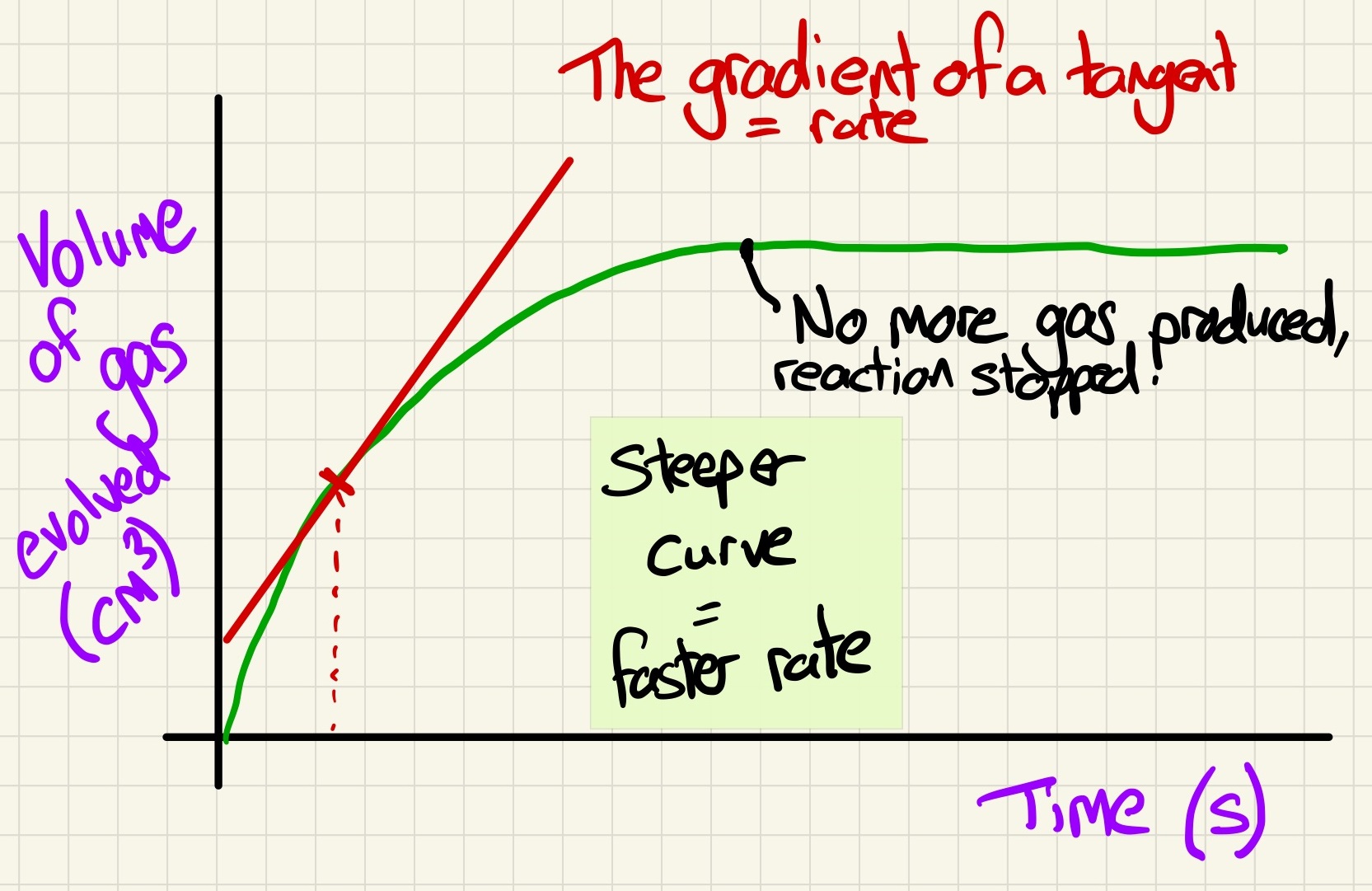
Look at the ways that we measure the rate of a reaction and how it can be calculated using graphs and tangents. The rate (or change in rate) can be seen my the gradient of the curve.
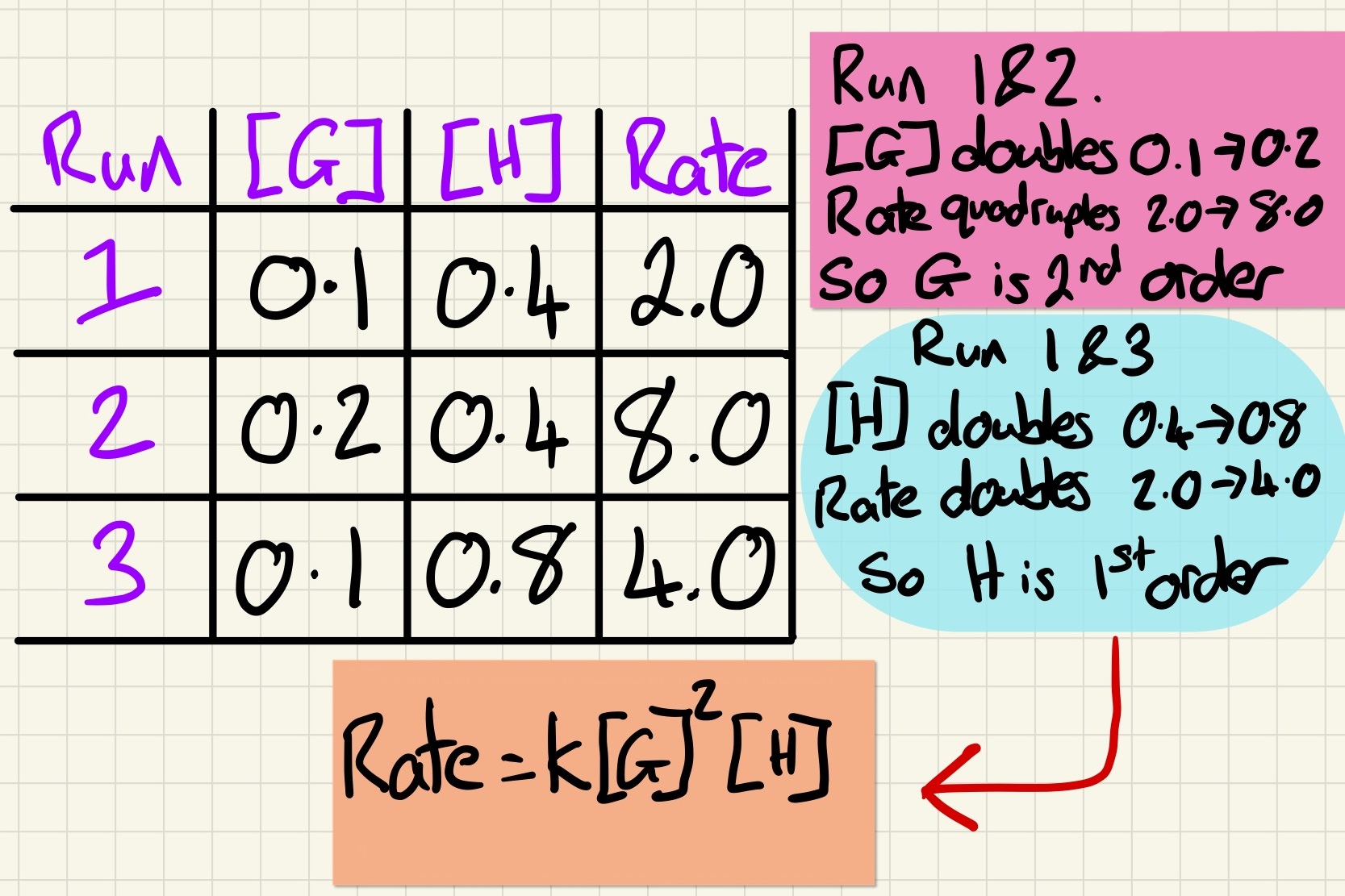
The order of a reaction affects the rate equation and the order of a reactant can be 1st, 2nd or 0. If a reactant is 0 order, it does not appear in the equation as anything raised to the power of 0 is 1. 1st order are raised to the power 1 and 2nd to the power 2. An example is:

Rate = k [A] [B]2

You can find the order of reaction by using experimental data. When [A] is constant, if [B] doubles and the rate doubles, then B is 1st order. If [B] doubles and the rate quadruples, the B is 2nd order. If changing the concentration has no impact at all, then the reactant is 0 order and will not appear in the rate equation. Rearranging the rate equation with this data will allow you to calculate k, the rate constant. Don't forget that the rate constant is only valid for that particular temperature.
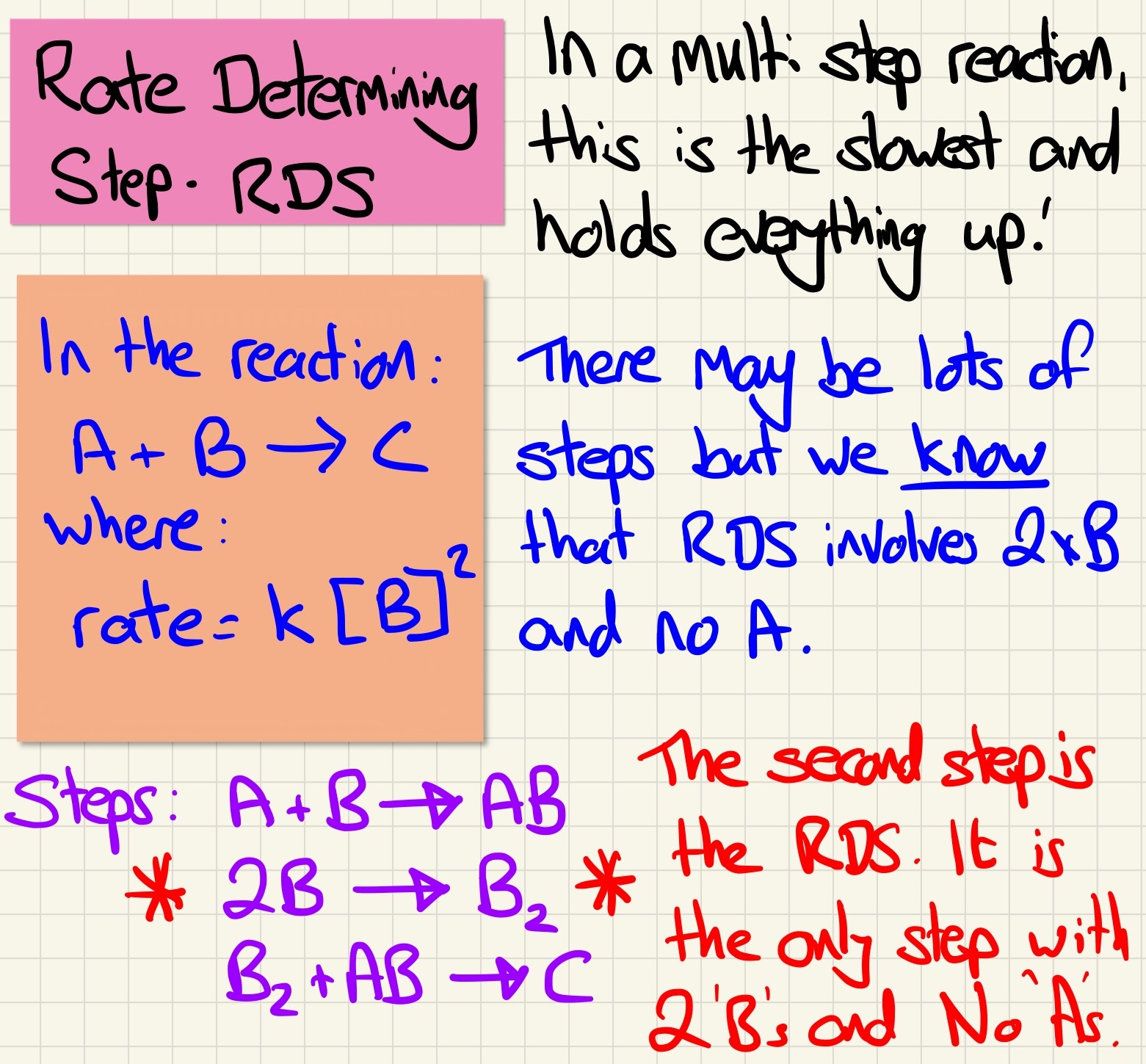
The rate determining step is the slowest step in a multistep reaction. This is the one that governs the overall reaction speed.
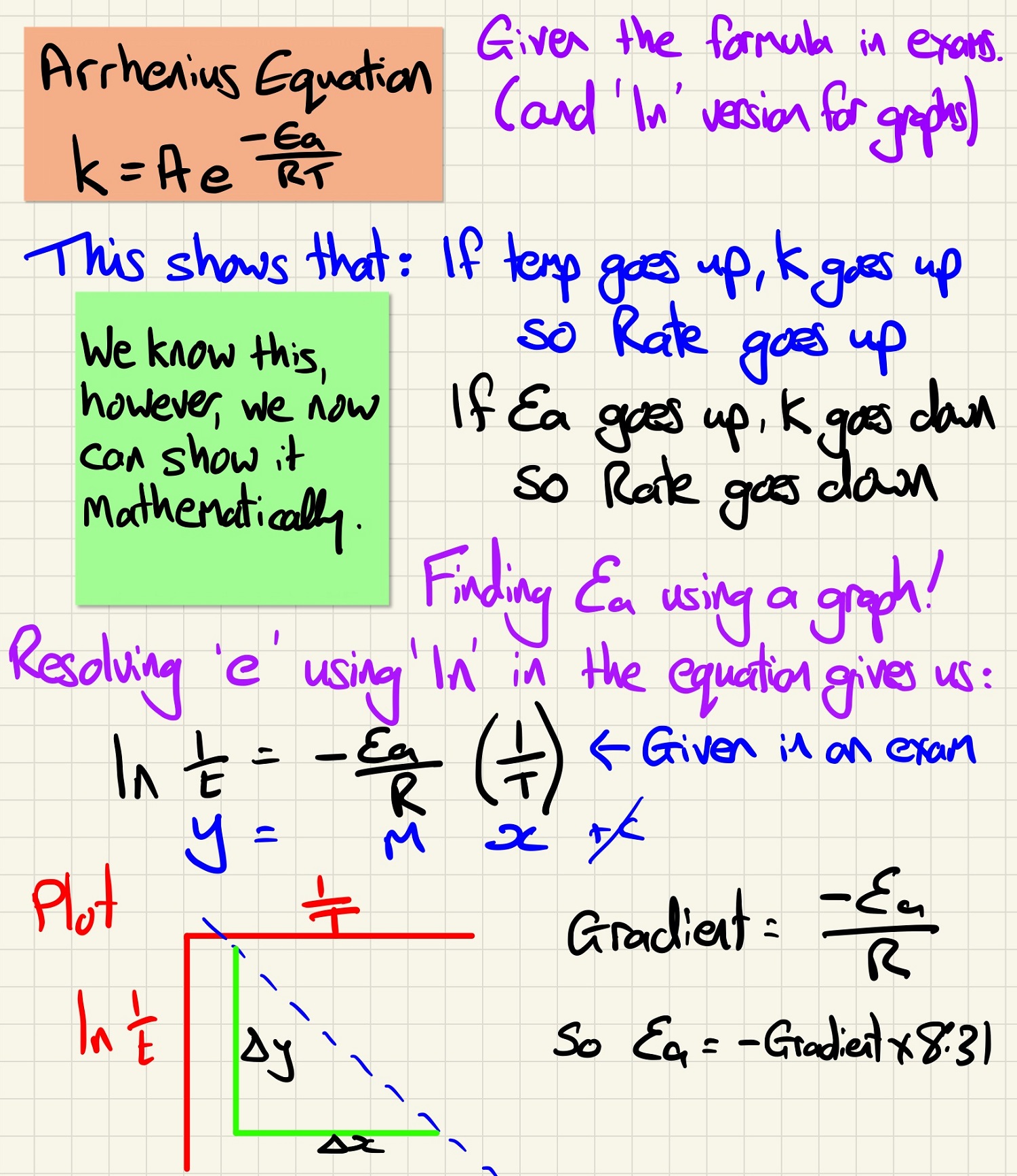
The Arrhenius equation seems complicated, however, you get it in the exam and the version from which you can plot a graph. Look at the note below and how it shows the relationship between rates, temperature and activation energy. You need to be able to find the activation energy from a graph.
This page was updated on: 9th November 2022
