A2 - Electrochemistry
Key learning for this topic
This topic builds onto the work that you did on REDOX. Before getting stuck into this module, take the time to ensure that you fully understand REDOX in terms of electron transfer, half equations, ionic and REDOX equations and that you can confidently balance REDOX reactions in acidic conditions. Have a look at the AS REDOX topic before you begin.

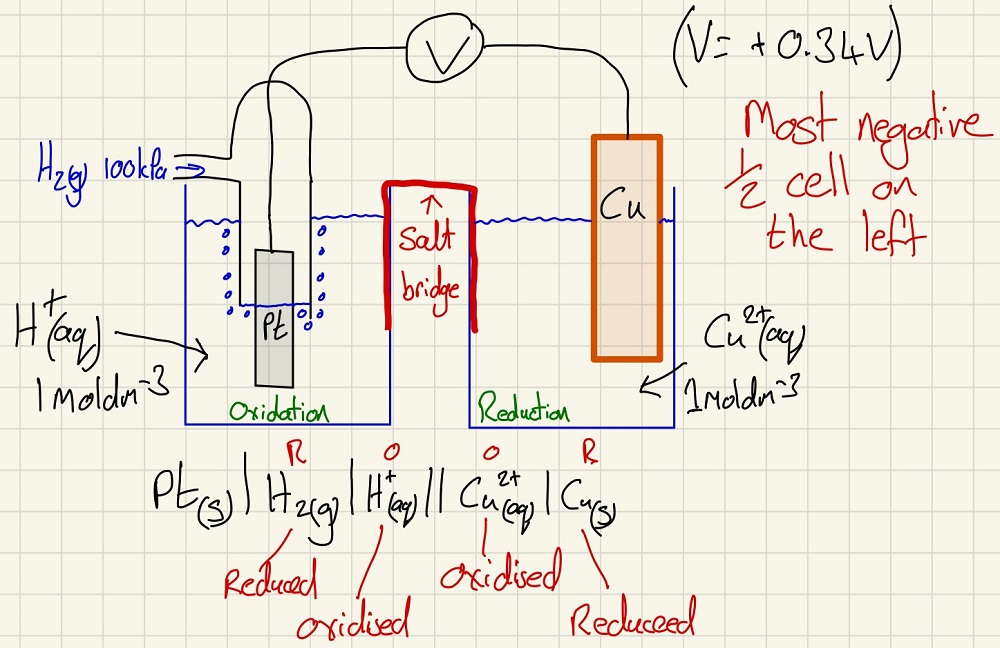
The electrochemical series is a way of showing reactivity in a quantitative way. Every redox half equation can be given a half cell potential. These are achieved by setting up an electrochemical cell against a standard hydrogen electrode. In this standard, we have a platinum electrode (chosen because it is the conductive interface but is so unreactive that it has no effect on the EMF of the cell) in a 1 moldm-3 of H+ ions with H2 gas being bubbled up through the solution onto the electrode at 100 kPa.

Below is a cell drawn out and it has the standard cell notation below it. Note that we put the most negative half cell on the left, this way, Reduction happens on the Right. The differences between the half cell values is the overall EMF of the cell. Single vertical line = phase change. Double vertical line = salt bridge and when there are two species both in solution, they are separated by a comma.
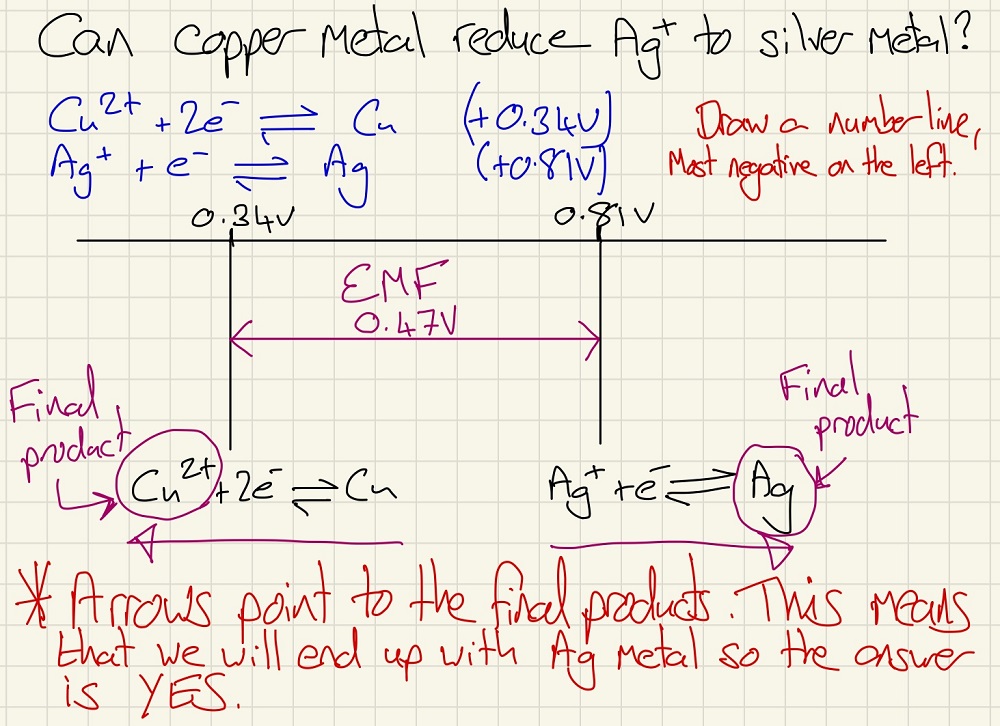
Another use for this data is to see if a reaction will take place. If you add your half equations to a number line, as shown below, you can firstly work out the cell EMF. Secondly, be drawing the arrows moving from the centre outwards, you can see what the final products will be if they were mixed. You may be asked if one species can oxidise or reduce another, this is the perfect way to work this out.
Commercial cells work in exactly the same way, they have two half cells that are connected by a conductor (instead of a salt bridge) and to replace the high resistance voltmeter (used to prevent the flow of a current) we have a device which will utilise the flowing current. Depending on the combination, different voltages are achieved. Zinc-Carbon = 1.5V, Alkaline =1.6V, Lithium ion (for phones) = 3.6 V and so on. Some are rechargeable where as others need to be recycled.

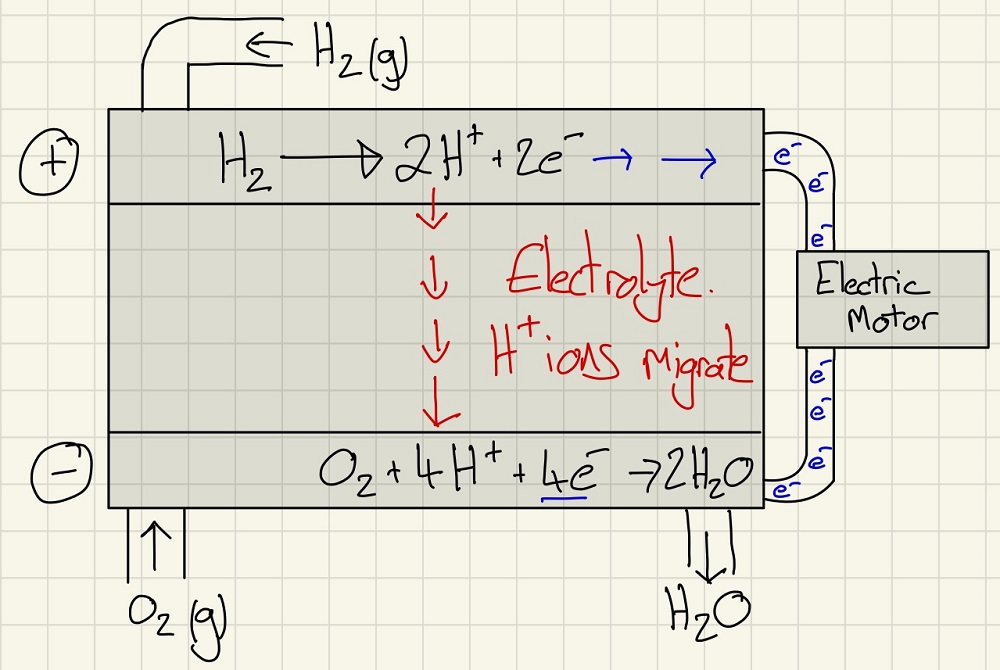
Hydrogen-Oxygen fuel cells work by bypassing combustion but harnessing the electron transfer when hydrogen reacts with oxygen to make water. This way, there is no energy lost as heat and the only exhaust product is water. Amazing!!! So why doesn't every car, bus and lorry have these? Simply put, it is brilliant, however, in order for this to work, we need hydrogen and the only way to make it currently is to electrolyse water and this requires electricity generated by fossil fuels.
This page was updated on: 19th November 2023
