A2 - Transition Metals
Key learning for this topic
Before beginning this section, go back to electron structure and ensure that you can remember how the transition elements' shells are filled. Simple things like: 4s fills first and empties first and that Chromium and Copper half fill the 3d so only have 1 electron in the 4s orbital will allow you to understand this topic much better.

Transition metals must have a partially filled d orbital when a metal or an ion. For this reason (and others that we will see) Zinc is not a transition metal. Transition metals have a variable oxidation state, As you can see from the diagram, some have more than others and those with many do have preferred (more stable) states too.
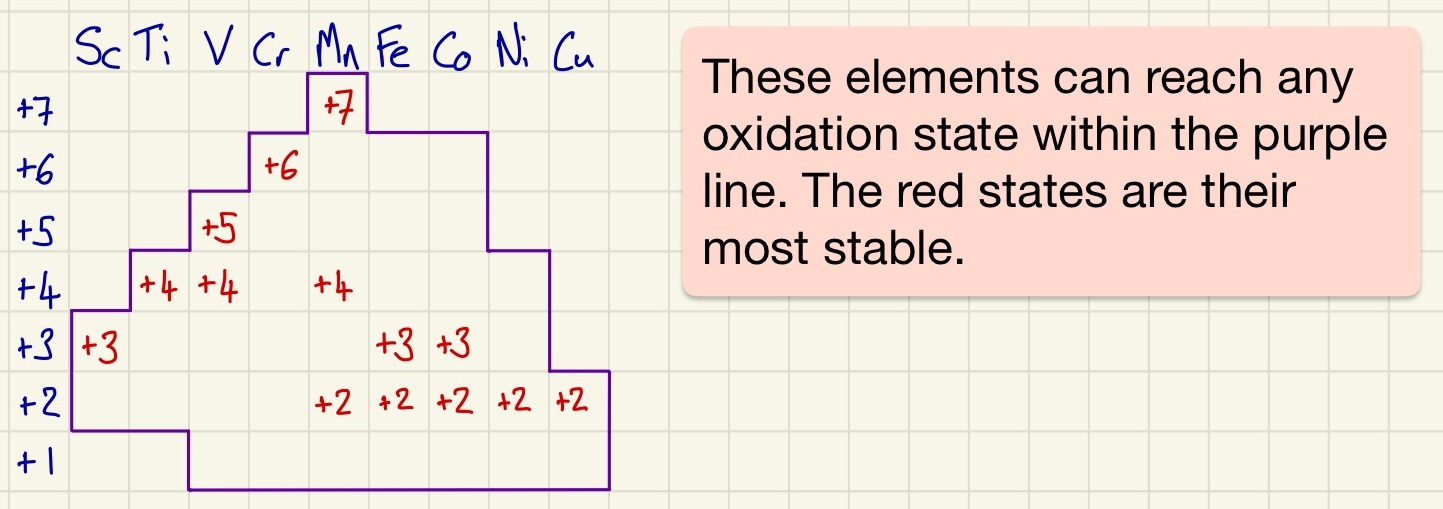
When in solution, transition metals form complexes. A complex is a metal ion surrounded by ligands, each ligand donates 1 or more pairs of electrons (forming a coordinate bond) to the metal ion. Complexes have 2, 4 or 6 coordinate bonds, the number of bonds is called the coordination number of the complex. Ligands can be unidentate (form 1 coordinate bond) like H
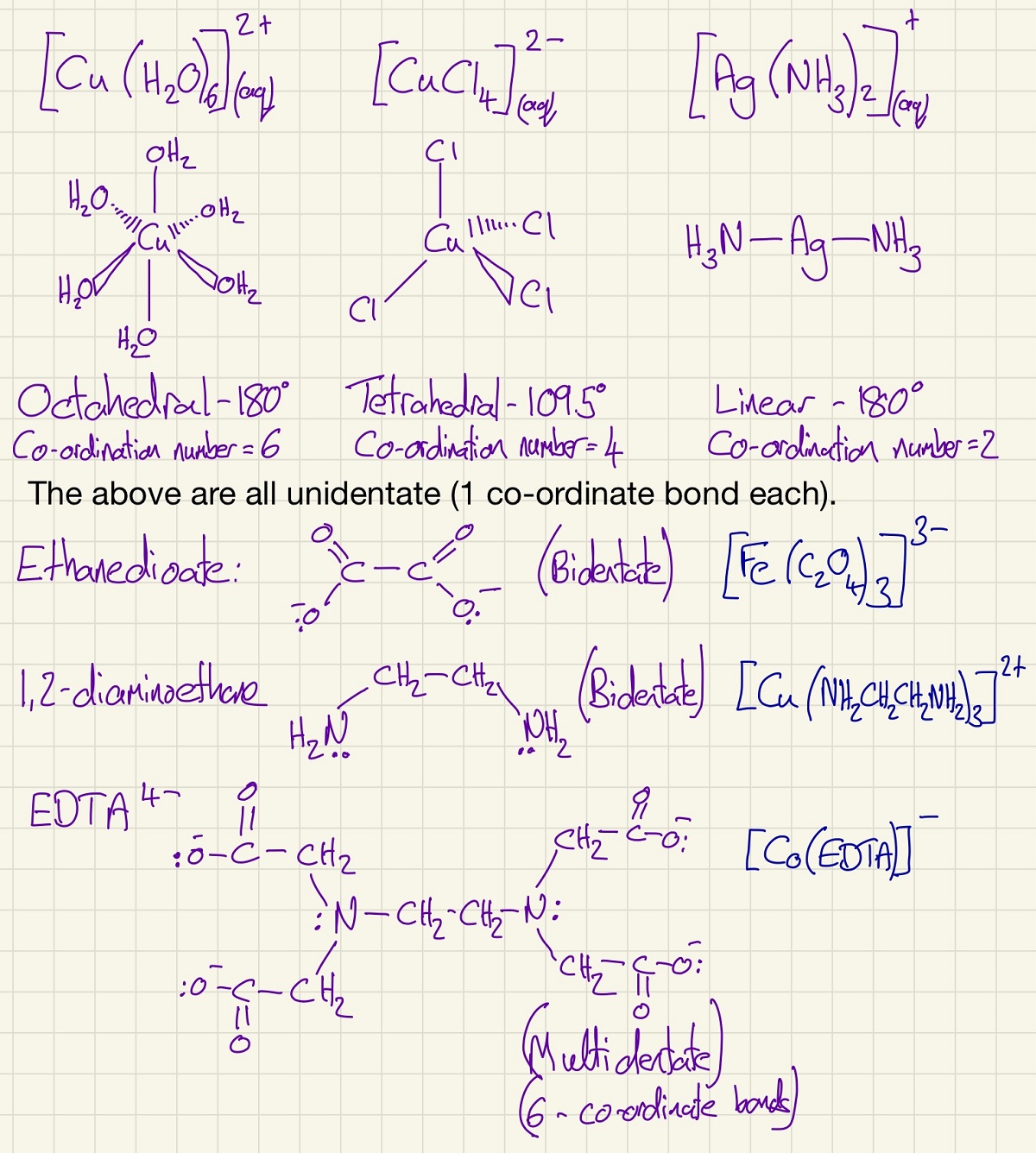
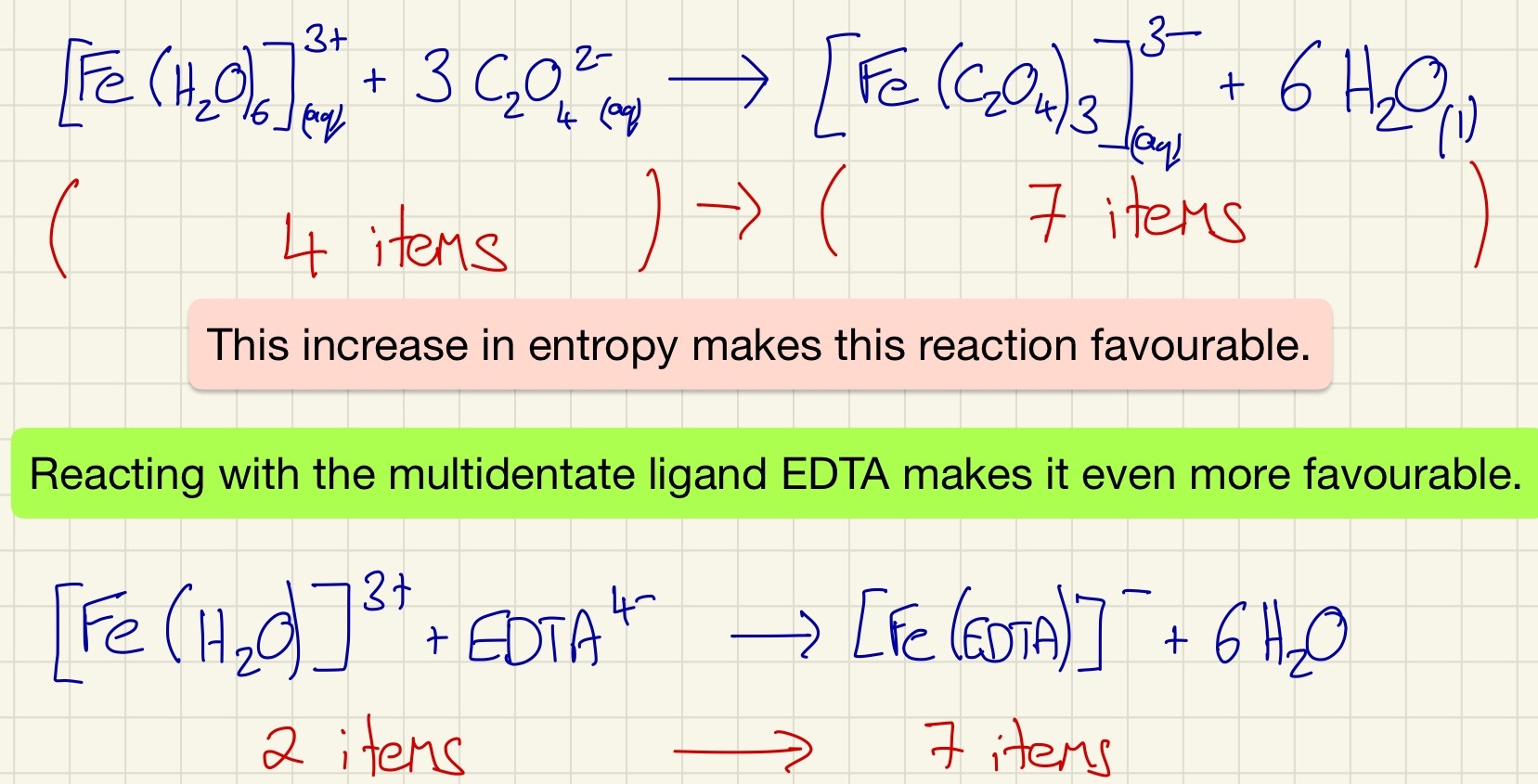
The enthalpy of a ligand substitution reaction is so small that it is negligible. Swapping one coordinate bond for another has very little impact. The biggest difference is when there is a change in entropy (Δ S). By swapping 6 unidentate ligands for 3 bidentate or a single multidentate ligand causes an increase in entropy which will make the reaction favourable as Δ G is now negative. This is particularly useful if somebody swallows a toxic substance like lead ions. By substituting the 6 waters with EDTA (this happens because there is an increase in entropy), the lead is then safe as no single or bidentate ligand can remove the EDTA so it will not be able to react with the body.
Transition metals form coloured complexes, the reason that they form colours is because their d orbitals have distinctly different energy levels and by absorbing light, electrons can become excited and move to a higher energy level. By doing this, they are only absorbing a specific wavelength of light. The rest of the light is left and this is what we see. For example, as Cu2+ blue, this is because the ions absorb the red and yellow light and only leave behind the blue which we can then see. This is why Zinc (II), Copper (I) and Scandium (III) do not form coloured complexes, Zn2+ and Cu+ have full d orbitals and Sc3+ has an empty one.
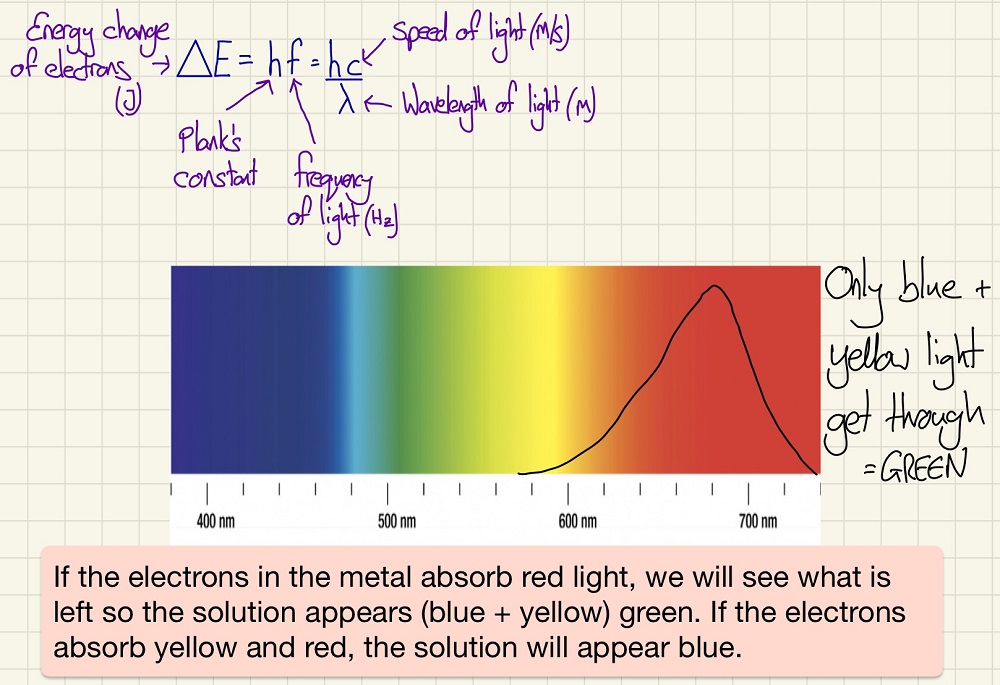
The colour of the complex can be affected by the oxidation state of the metal, the coordination number, the ligands and the metal itself. As these things are altered, then the difference between the energy levels is different which will mean that Δ E will change so the frequency/wavelength of the light absorbed will also change and so we see a different colour.

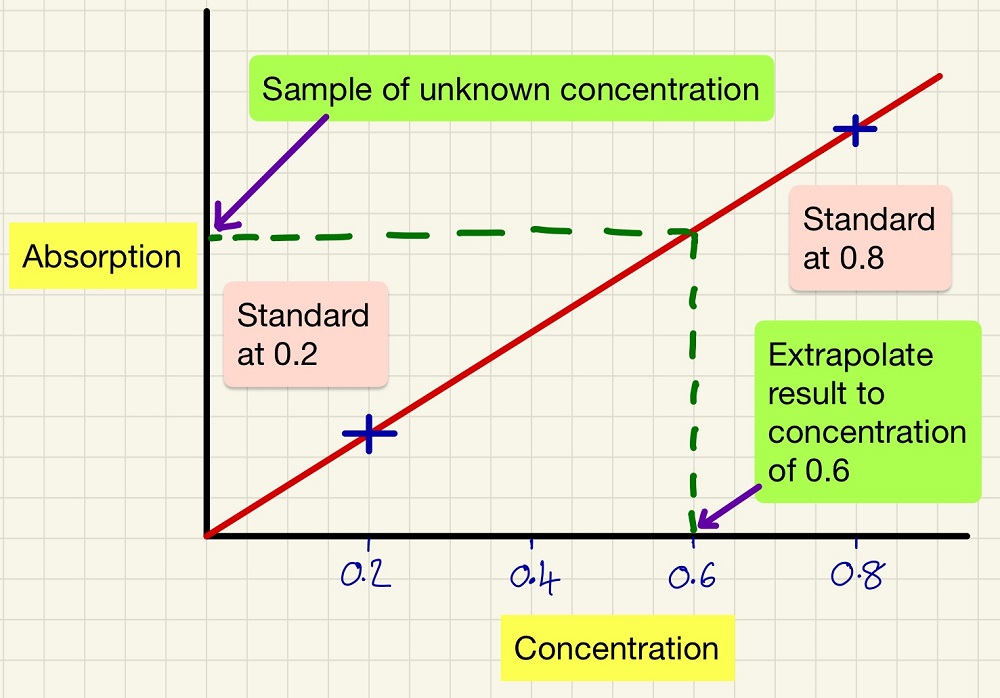
This is a very straight forward process that is very useful and has many industrial and medical uses. If you have a coloured solution and you do not know its concentration, you can use a colourimeter with a specific wavelength filter (to focus the measurements on a particular colour) to measure the absorption of two standard solutions. Plot these two on a graph as shown below. When you take a sample of solution later of unknown concentration, simply measure its absorption then read off the graph what its concentration is.
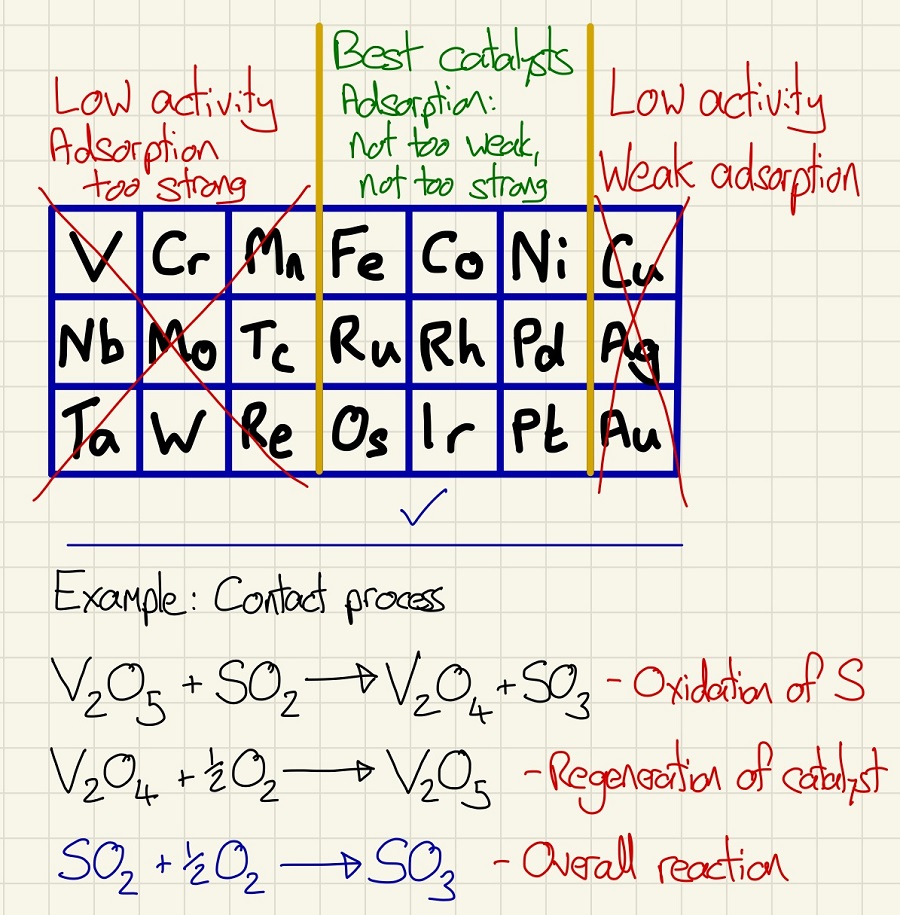
Transition metals have catalytic capability. Not all are suitable for use, for example, the highest levels of activity are around iron to nickel (and the elements directly underneath them) so these are most commonly used. Those to the right (Silver and Gold), these weakly adsorb substances onto their surfaces so the reactions cannot properly happen and suffer from low activity. Similarly, those to the left of Iron also have lower activity but they also adsorb too strongly so the reactants/products cannot desorb and become stuck to the surface. Their presence in the reaction lowers the activation energy.
This page was updated on: 6th November 2023
