AS - Introduction to Organic Chemistry
Key learning for this topic
I have struggled as to how to present this topic. I have been very tempted to split it up as it is so big but for now, I am keeping these together as they are so closely interconnected. As with many other topics, this is built upon knowledge that you have gained during your GCSE studies. Because of this, prior to starting this topic, please revisit the Organic Chemistry section from last year. We assume that you are already fluent in the processes of fractional distillation, catalytic cracking, combustion, incomplete combustion, atmospheric pollutants and carbon neutral fuels. This list is not exhaustive so keep reading around the subject.

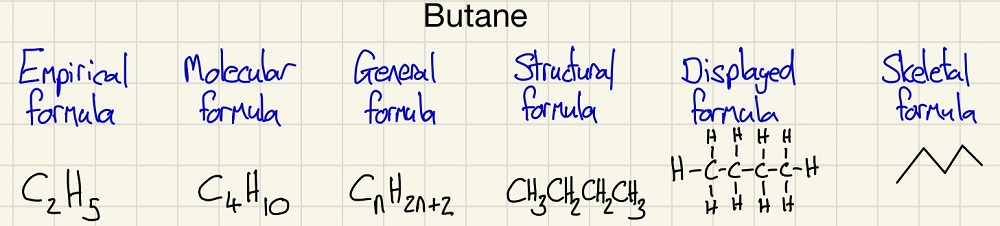
We will begin with the different types of formulae used in organic chemistry. It is very important to keep practicing them as I still get two of them mixed up now and had to look them up!
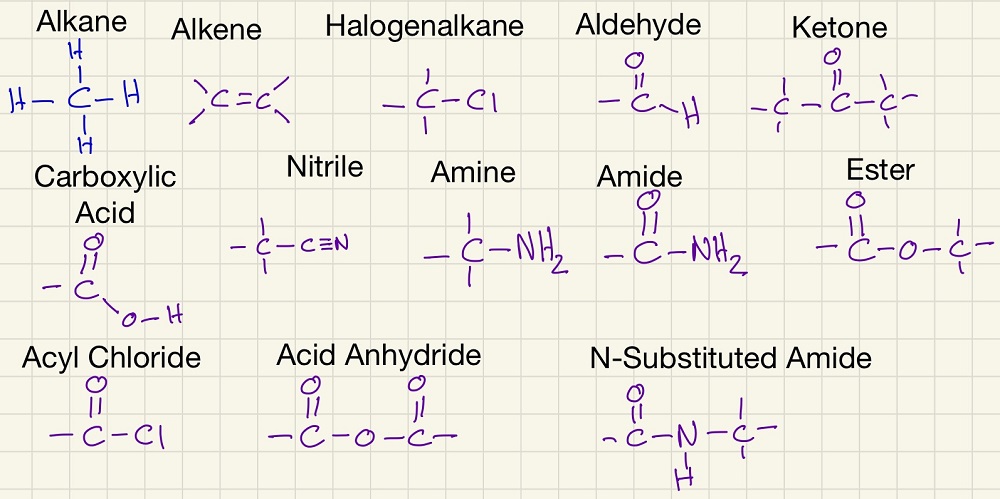
Next, you need to be able to recognise the different functional groups that you will come across. We will meet them all again so don't worry about it yet.
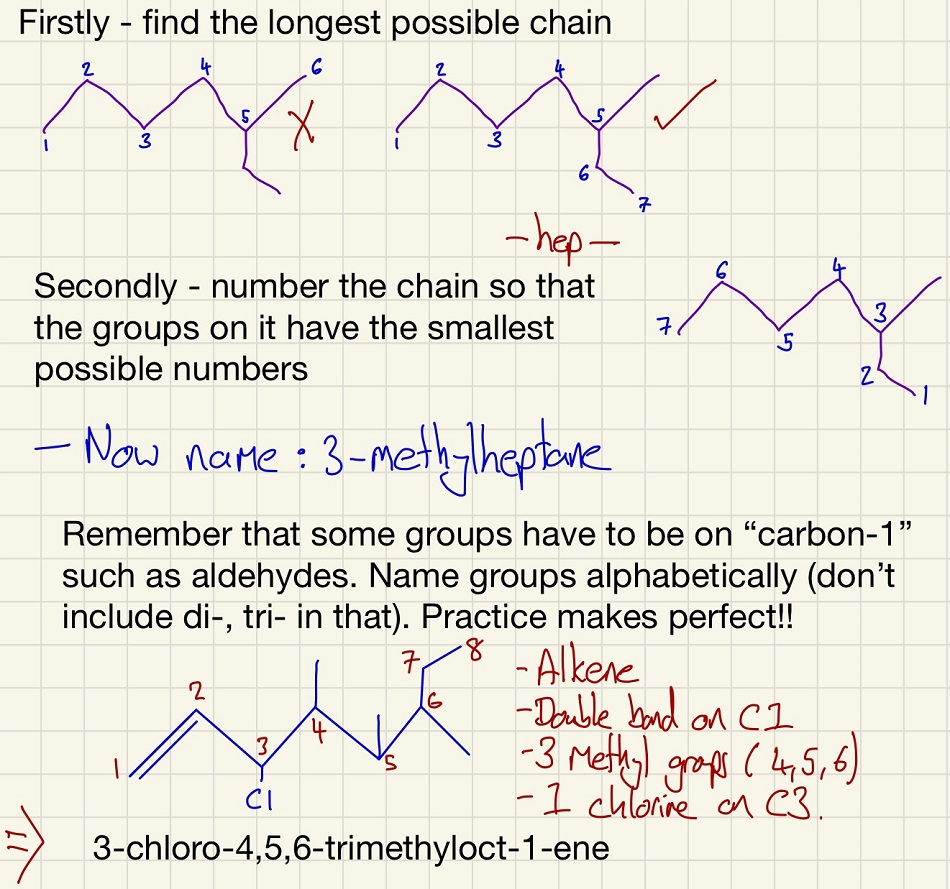
The next thing to look at is the naming of compounds. I could write a whole section just on this but I will, for now, share a few rules? Please go and find some videos on YouTube that make sense to you or different sites that talk you through this process. Let me know them and I can add them to the list of links. During your studies, whenever you see an organic molecule, work out its name then check the answer. This will be the best way to learn and keep the skill fresh in your head. Always refer to IUPAC names.
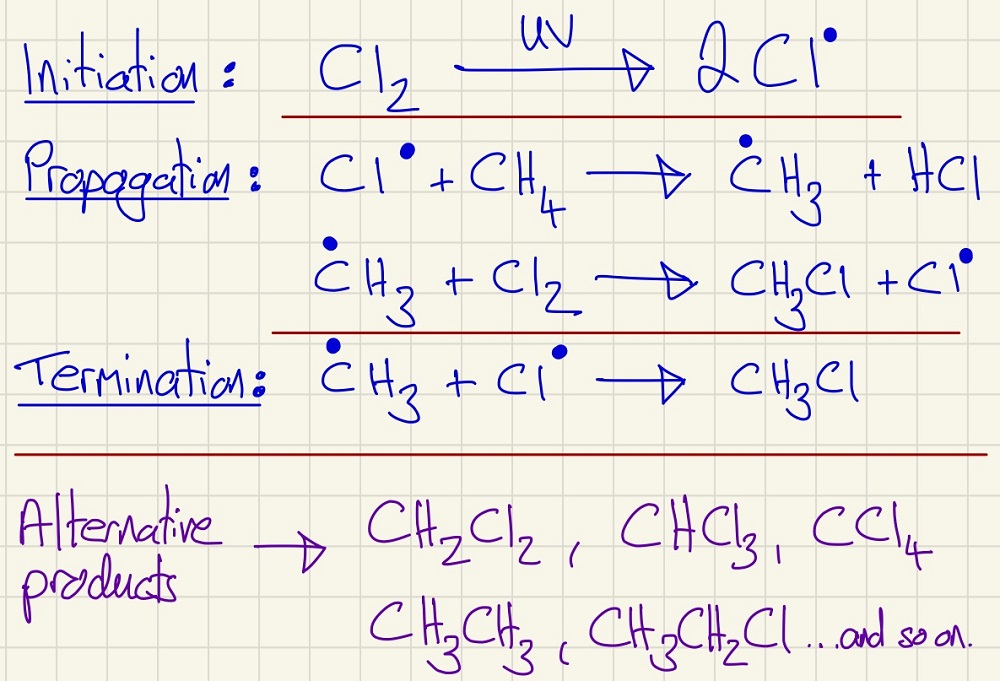
Free Radical Substitution. This is the first type of mechanism that we meet. You need to be able to reproduce these from memory and apply them to different molecules etc. Free Radicals are exceptionally reactive species, they are formed when a covalent bond is broken through homolytic cleavage. This means that each side has a single (unpaired) electron. When these react, they leave a new species with this burden. The radical is drawn with a single large dot over the atom that has the single electron. Note all of the steps in this reaction and you will see the huge variety of potential products, although you will be asked to focus on one in particular.
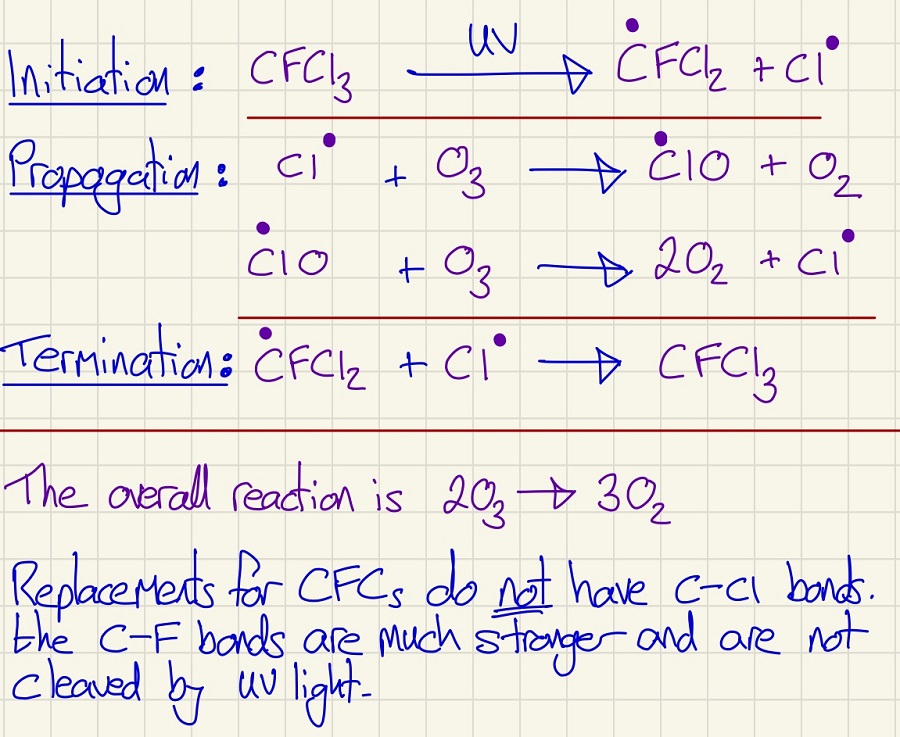
Here is another example of Free Radical Substitution and it has been happening for decades in the upper atmosphere and it is depleting the ozone layer. The reactions of these gases are the reasons why refrigerators have to be carefully disposed of, why gases in a car's air conditioning are collected and not released and why aerosols from deodorant to inhalers have had their propellants changed.
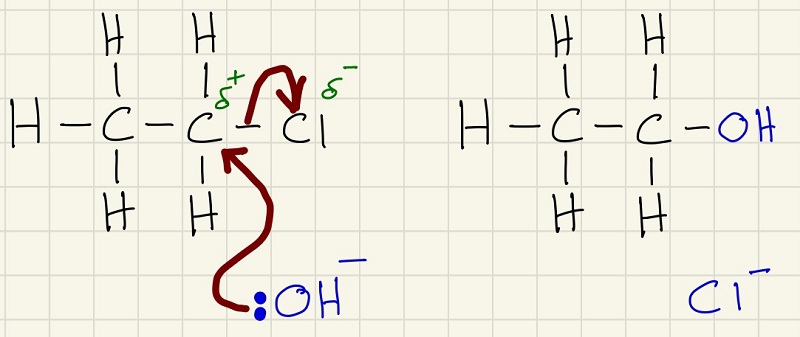
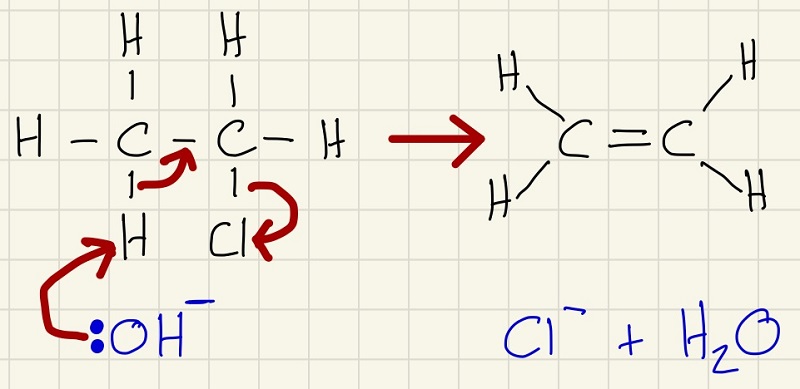
The first reaction of a halogenalkane is the nucleophilic substitution reaction with KOH.
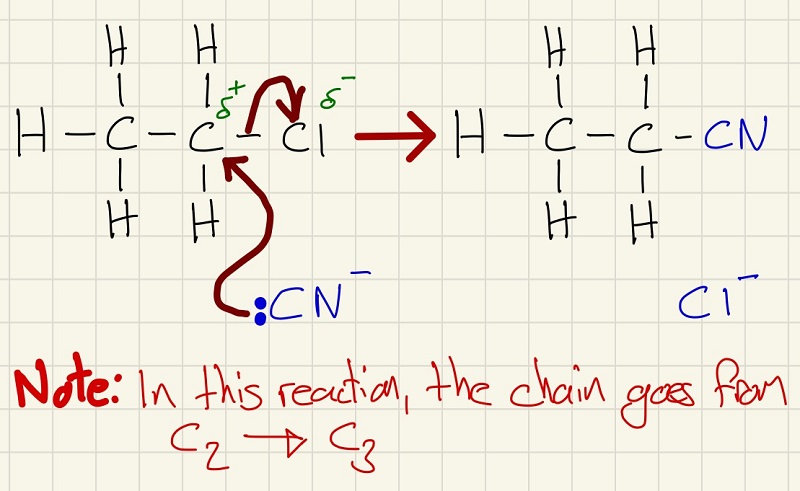
The next is remarkably similar, this time the reactant is KCN. (Note the effect on the length of the chain)
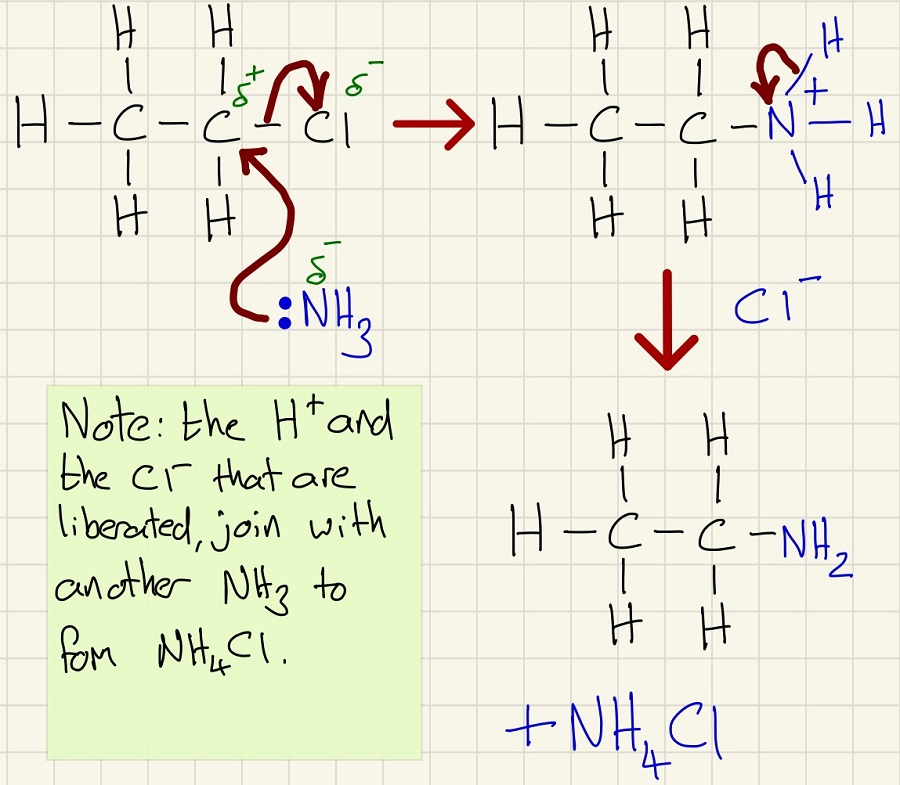
Thirdly, another nucleophilic substitution reaction, however, with using the uncharged ammonia molecule (NH3), there is an extra step
Another reaction involving the halogenalkanes is elimination. This produces an alkene and you need to be able to reproduce the mechanism.
There are several types of isomerism - same molecular formula but atoms rearranged differently:

• Chain- the carbon chain is different

• Position - the group is on a different carbon (1-chloro instead of 2-chloro)

• Functional group - aldehydes & ketones, alkenes & cyclic alkanes, Carboxylic acids & esters

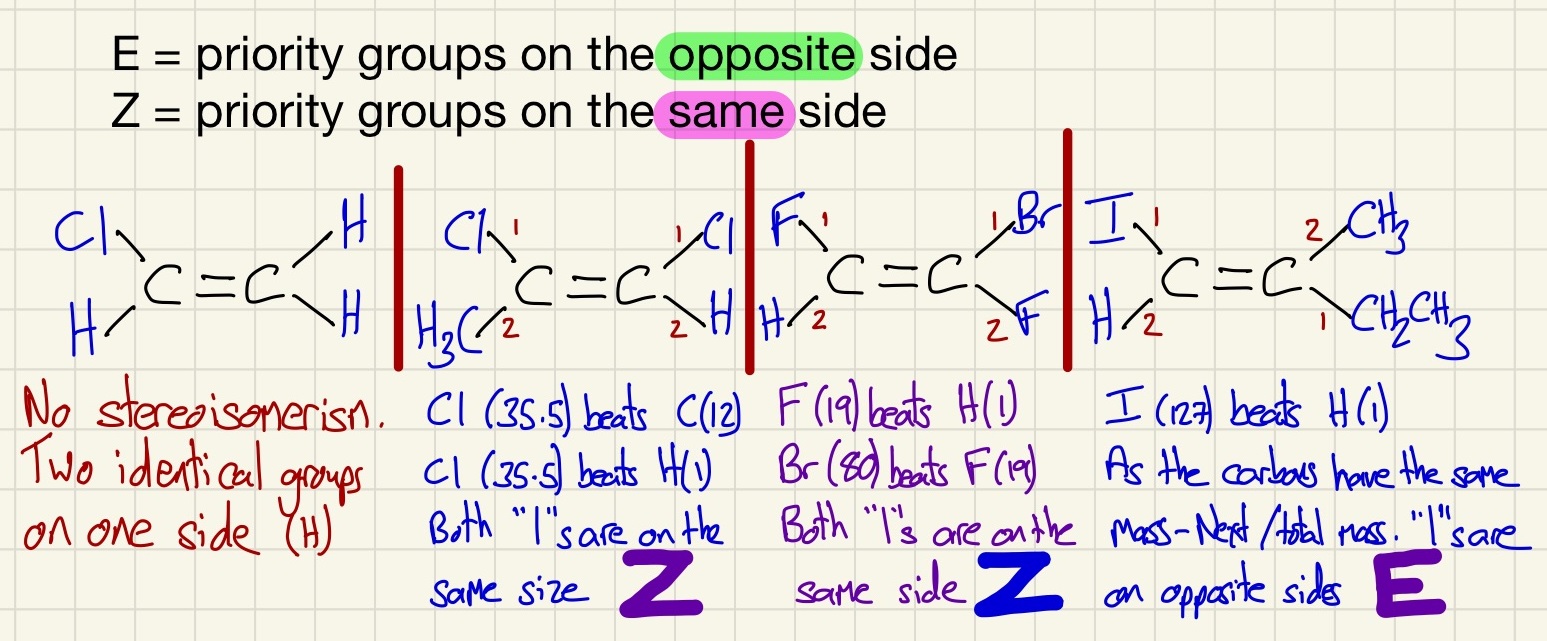
A different type of isomerism which is exclusive to alkenes is Stereoisomerism. Because there is no free rotation of the C=C, alkenes with a pair of different atoms attached to the carbons that are double bonded can be very different.
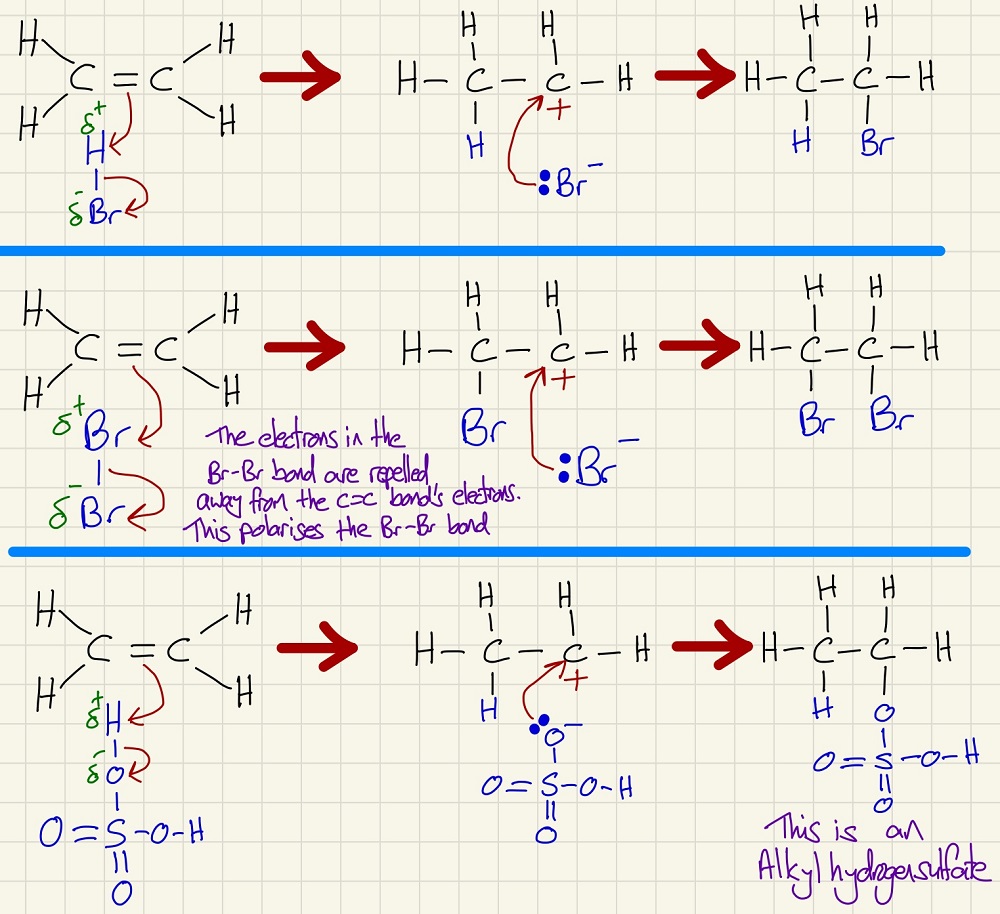
More mechanisms - these three are all electrophilic addition to alkenes, you need to know, understand and be able to reproduce these.
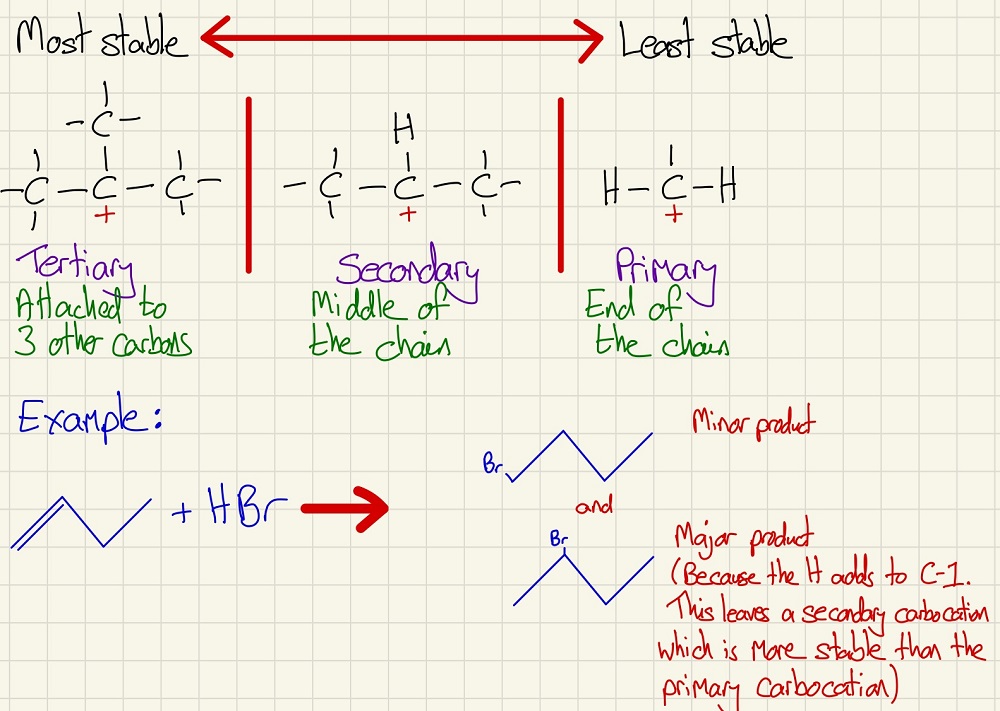
The addition of HBr to alkenes with 3 or more carbons can give multiple products depending on where the H and the Br add. The stability of the intermediate carbocation (C+) determines the major product.
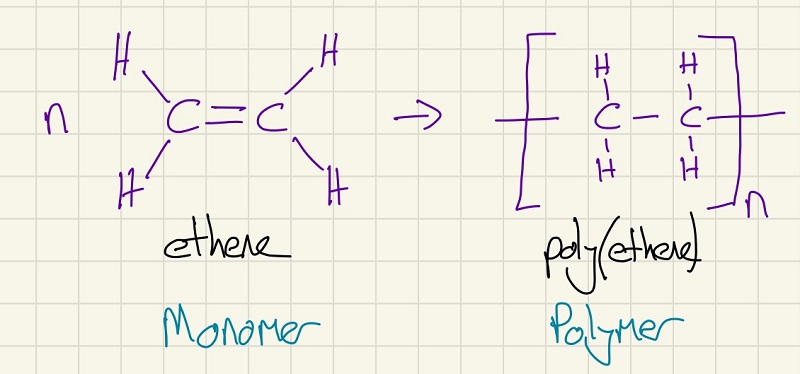
Going back to GCSE, you need to remember that alkenes can be polymerised. Make sure that you have a look back and note that the alkene can greatly affect the properties of the polymer.
This page was updated on: 4th November 2023
