AS - Alcohols & Organic Analysis
Key learning for this topic
There are two ways that ethanol is manufactured commercially. The first is through fermentation and the second is by reacting steam with ethene. You must be able to consider the environmental factors of both of these processes.

Ethanol can be made very easily by fermentation, this is a process that humans have been doing for 9000 years. More recently, ethanol has been used successfully as a biofuel. It can partially replace petrol in cars. This lowered the net increase of carbon in the atmosphere and reduced the reliance of oil from other countries. This is not used anywhere nearly as much as it was because growing sugar cane for fuel takes up land that is crucial for growing food for an ever growing population.

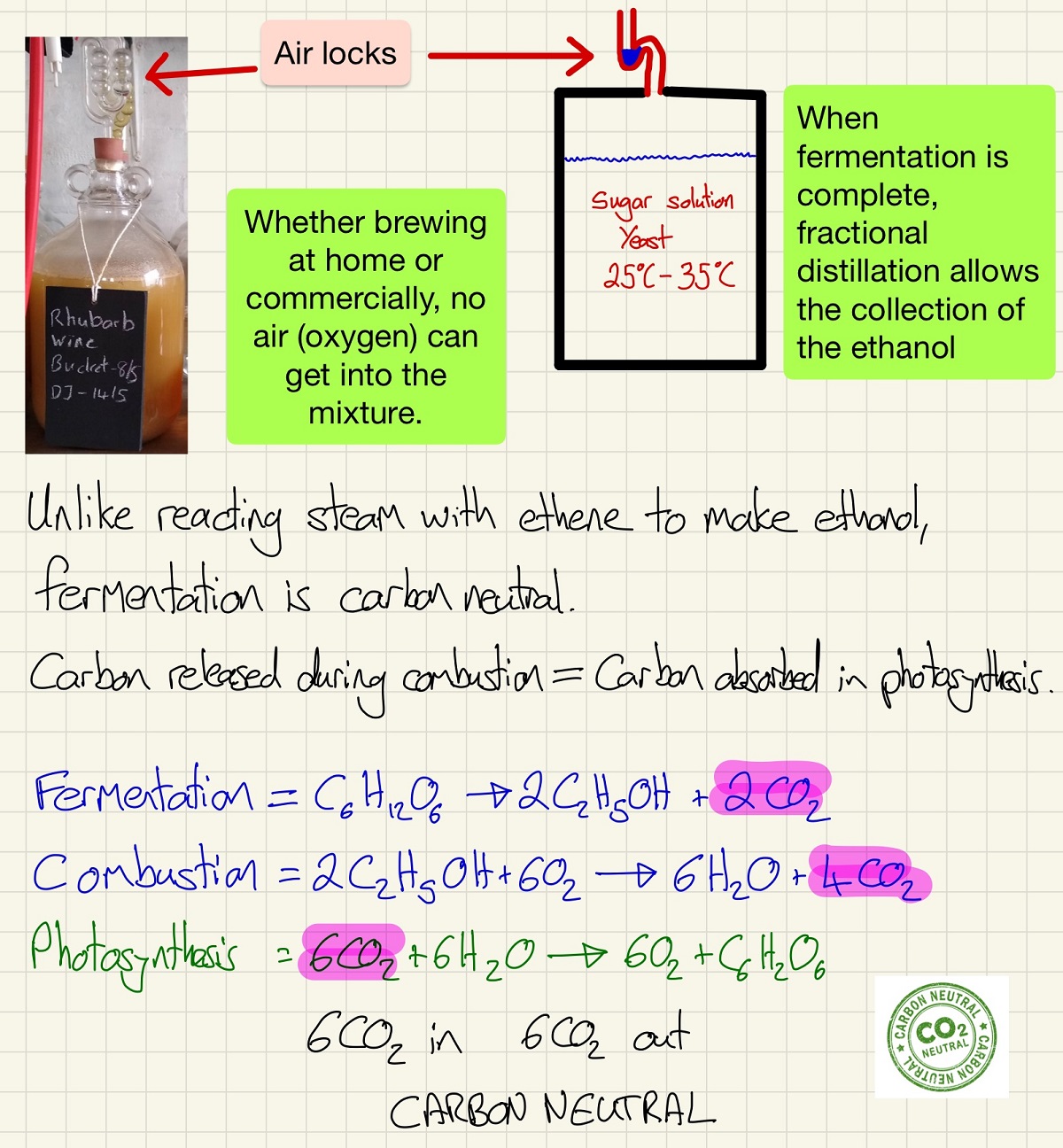
Below is a very simplified view of the fermentation process. The picture of the wine fermenting is from my own garage and the simplicity of this process is reflected in industrial processes too. Keep unwanted microorganisms out to protect the yeast and keep out oxygen to prevent the oxidation of ethanol as it is produced. You also need to be able to explain why fermentation and the combustion of the produced ethanol is carbon neutral.
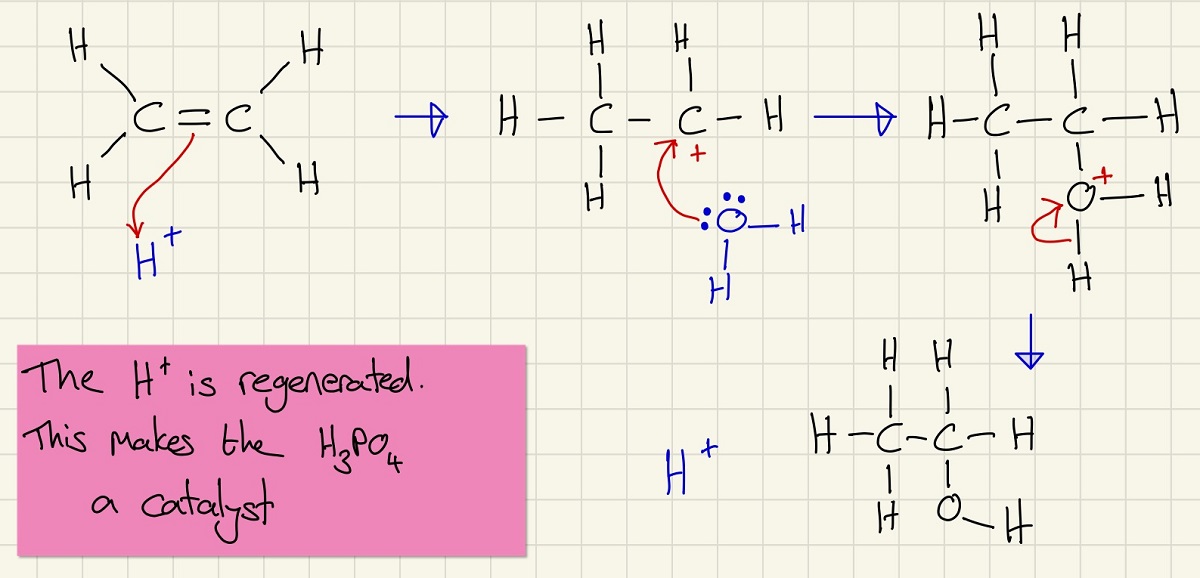
The next mechanism, you have already met. It is worth revisiting this process before we work through the reverse.
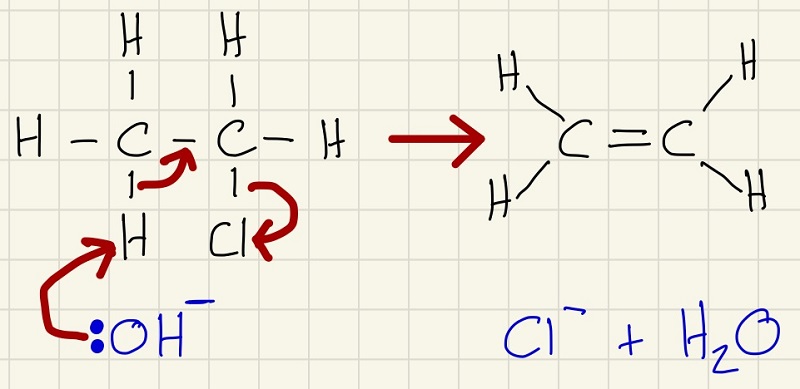
This is the mechanism for the hydration of an alkene.
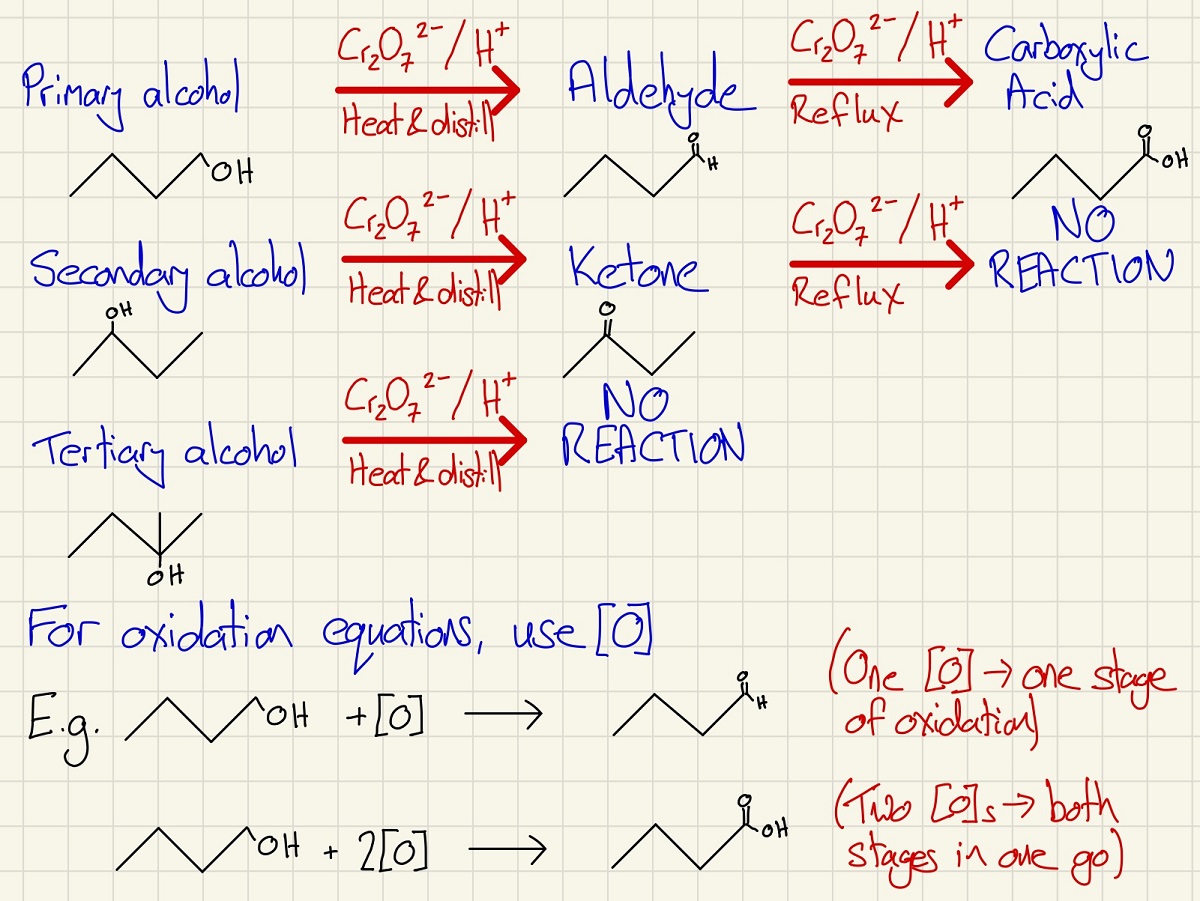
It is very important to learn the reagents used and the potential products during the oxidation of alcohols. Depending on their structure (primary, secondary or tertiary), different products (or none at all) are made.
Fehling's solution and tollen's reagent are both tests to distinguish between aldehydes and ketones.
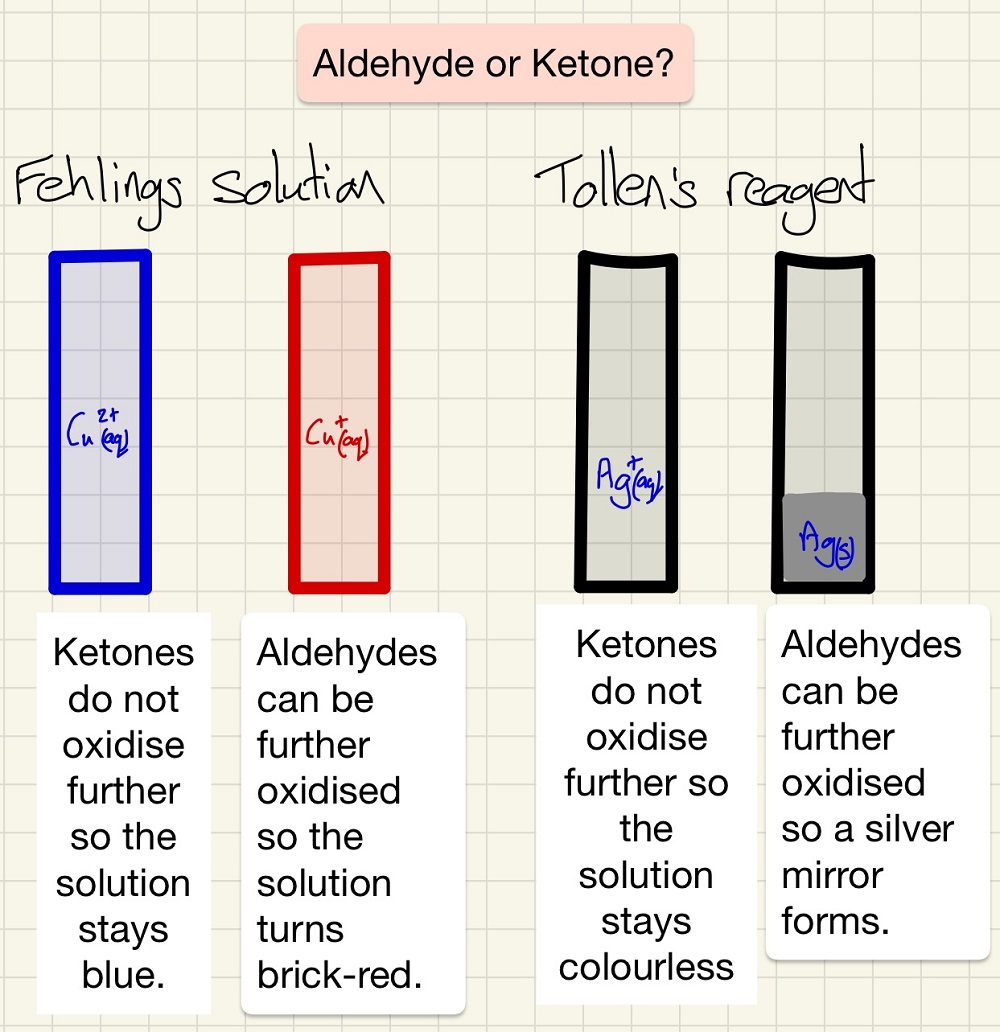
On top of the two tests above, you need to know that the reaction discussed earlier is actually a test too. By adding acidified K2Cr2O7 (orange) to an alcohol and warming it, it will turn green if the alcohol is primary or secondary and it will stay orange if it is a tertiary alcohol. Finally, if you produce a carboxylic acid, it will effervesce with sodium carbonate solution.

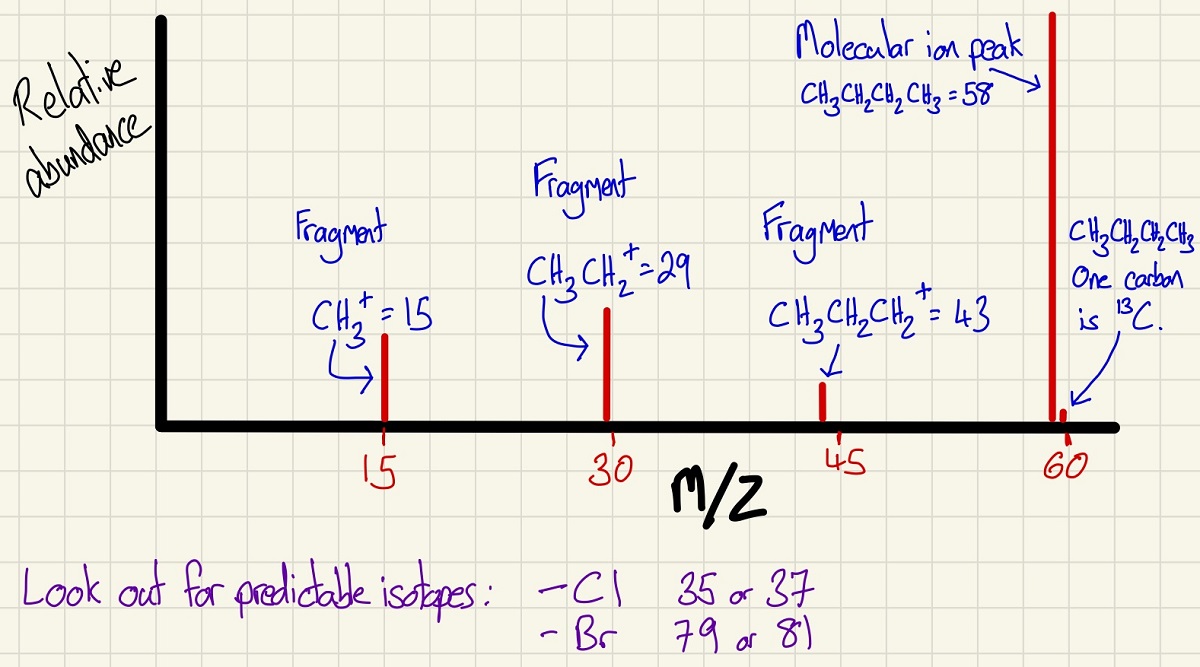
In the very first topic, we looked at Time of Flight Mass Spectrometry. In this topic, which includes chemical analysis, we look in more detail at mass spectrometry. Below is an example trace and it is really quite straight forward. The largest peak on the right is the Molecular Ion Peak and this is the mass of your molecule. There may be some smaller peaks close by and these are caused by different isotopes of usually the carbon but there may even be a deuterium there too. Look out for the other tell-tale isotopes that can indicate a chlorine, for example. If there are two peaks with a separation of 2 and the smaller one is 75% and the larger 25% as these show the ratio of Cl-35 and Cl-37. Other clues can indicate more about the structure, for example, in the one I've made below, the butane has fragmented leaving different sections. This helps if you can spot a carbonyl group etc. On its own, mass spec is not good at identifying a molecule, however, it works brilliantly when used in t
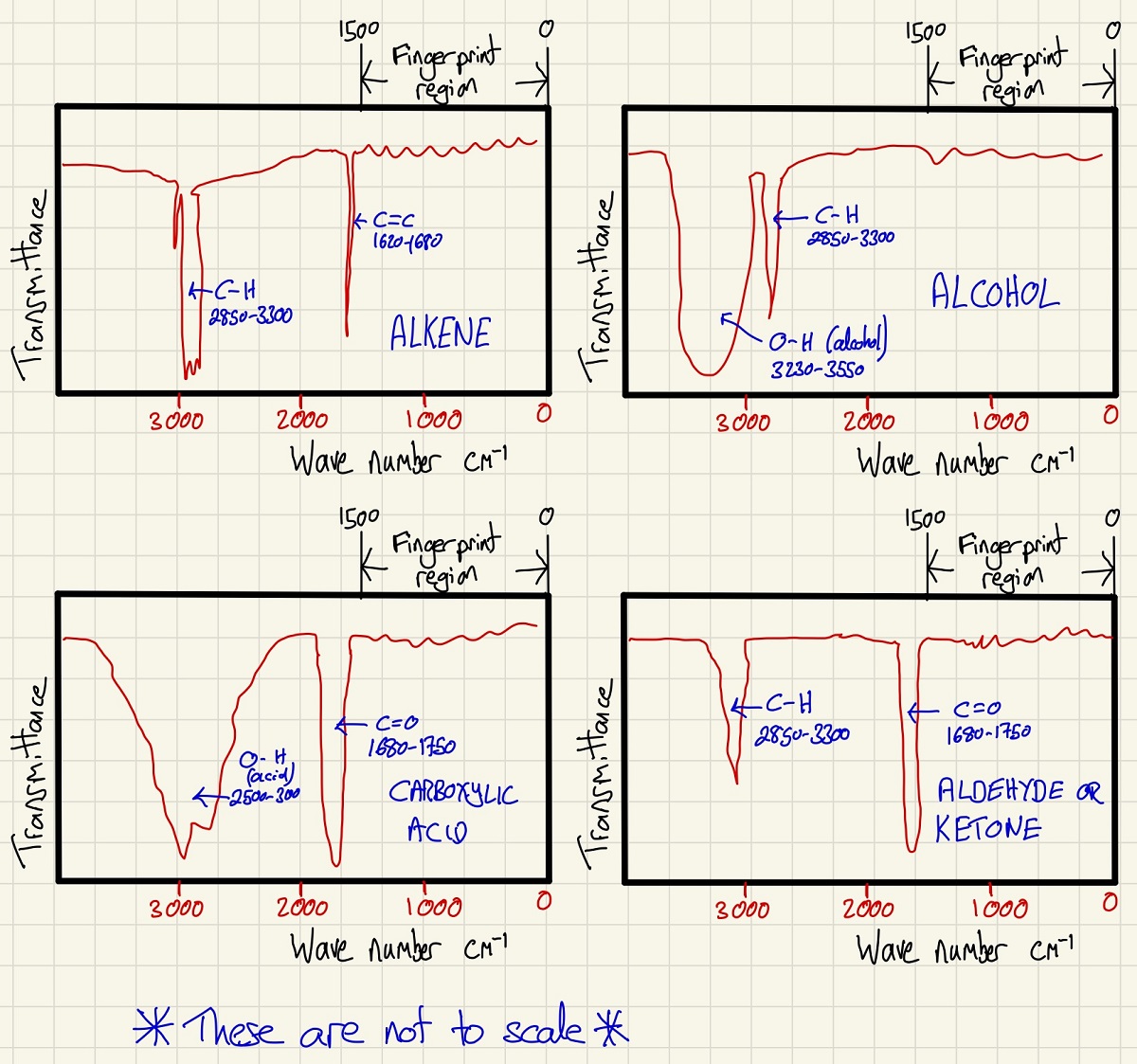
We know that photons excite electrons, in this method, we take advantage of the fact that bonds absorb energy and different bonds absorb different wavelengths of infrared (wavenumber is 1/wavelength). By simply reading the displays and identifying absorption to the left of the fingerprint region, we can identify functional groups that are present. Again, quite useless on its own at identifying a completely unknown molecule, however, in conjunction with Mass Spectrometry, you can find out a lot about the molecule and identify it.
This page was updated on: 4th November 2023
