AS - Periodicity and Groups 2 & 7
Key learning for this topic
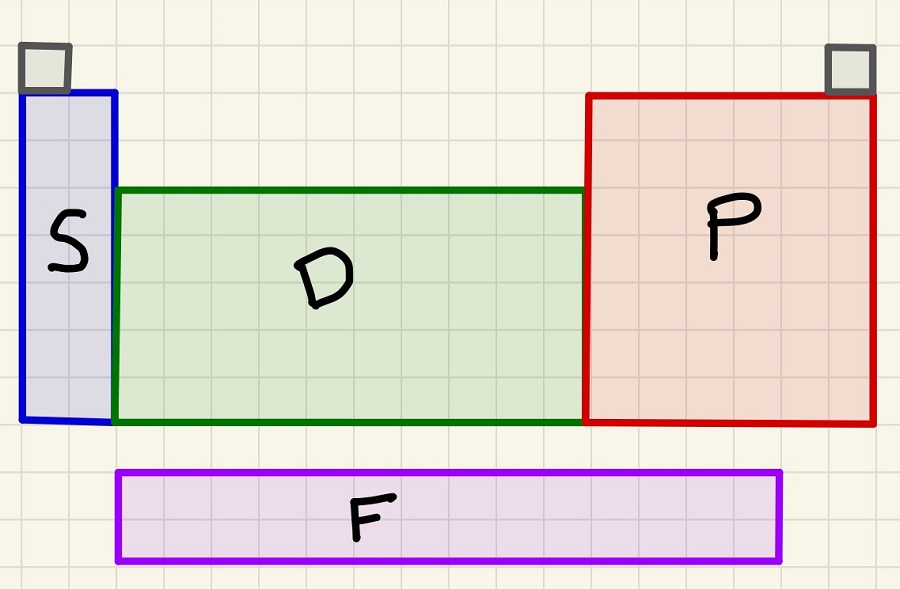
Do you know which block an element is classed as? Make sure that you can identify "S Block" or "D Block" elements
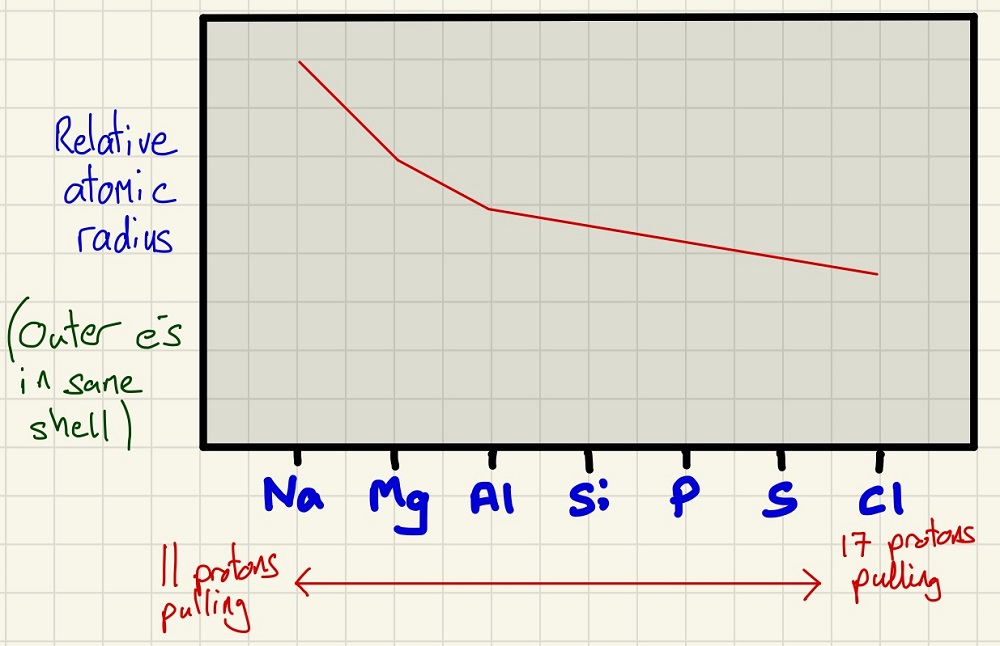
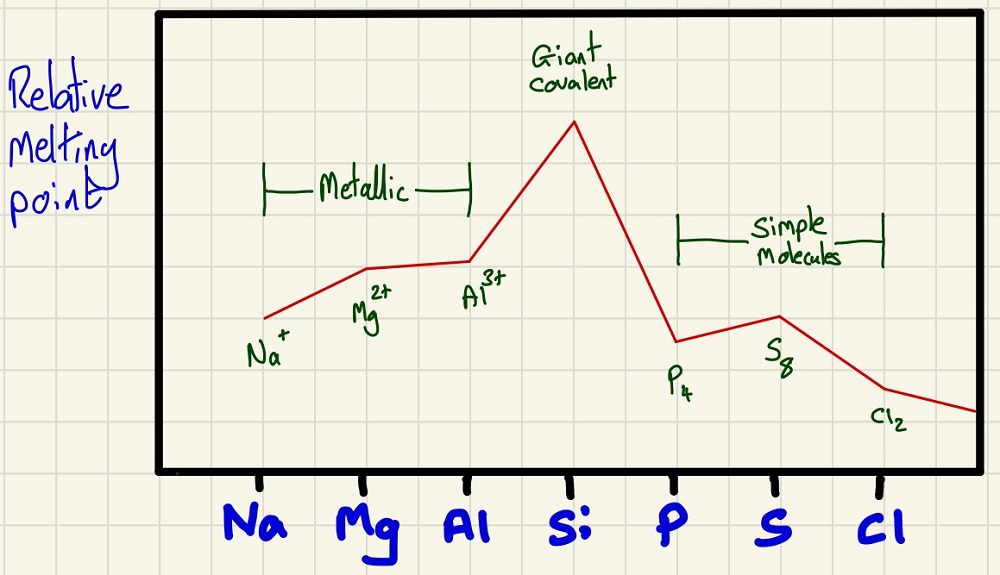
Below is the trend in atomic radius as you move across period 3 from Sodium to Chlorine. The trend is simply that as you move right across the period, the radius decreases. You need to be able to explain this (and understanding is even better), every time you move one element to the right, you add a proton which creates a stronger attraction to the outer electrons. All of the outer electrons are in the same shell so they are pulled in a little closer each time.
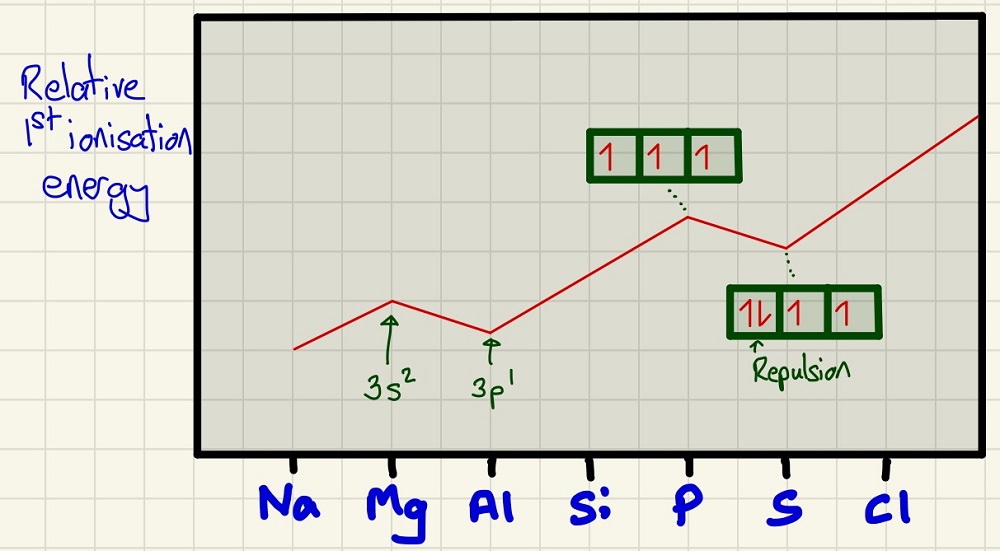
The first ionisation energy (the enthalpy change to remove 1 electron from each of 1 mole of gaseous atoms). Generally, it increases as, stated above, there are more protons so more attraction as you move to the right. There are 2 distinct anomalies, firstly, the dip from Mg to Al is because the Al electron is coming from the empty "P orbital" whereas the Mg one came from the "S Orbital". The second one is causes by having 2 electrons in the same orbital in S which adds some repulsion so one is "helping" to push one out when we are trying to remove it
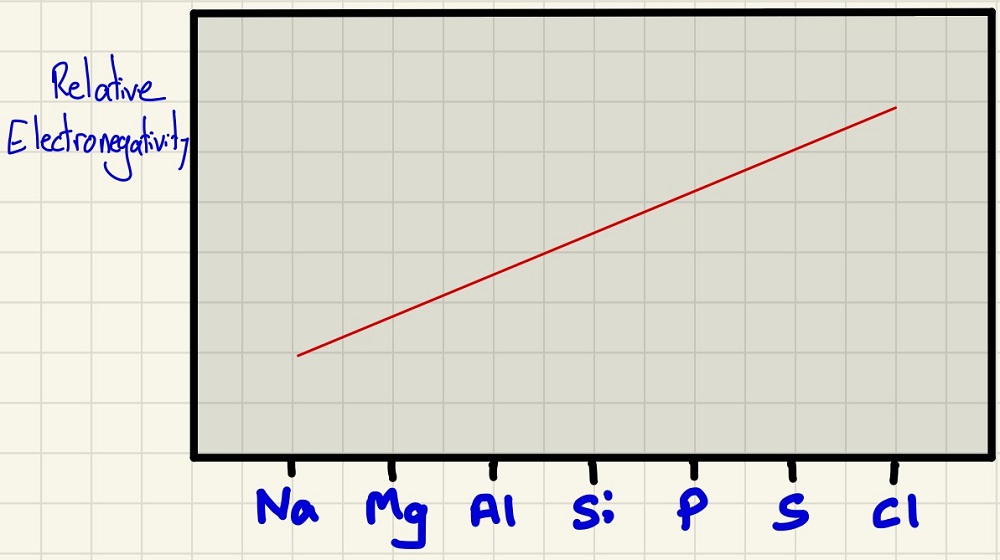
The electronegativity is very straightforward, linking to the atomic radius, as the atoms get smaller, the bonding electrons will be closer to the more highly charged nucleus so will be better able to attract the bonding electrons in a covalent bond towards itself.
The melting points are very closely linked to the structures of the elements. Na-Al are metals so have metallic bonds, as their charge increases, so does their attraction to the delocalised electrons around them. Si is giant molecular so has the enormous 3D lattice. The last three are all simple molecules to their intermolecular forces are Van der Waals' forces and are proportional to size. Here we need to know that P4 has less mass than S8 which is why it climbs for S. Finally, the lowest mass if for Cl2.
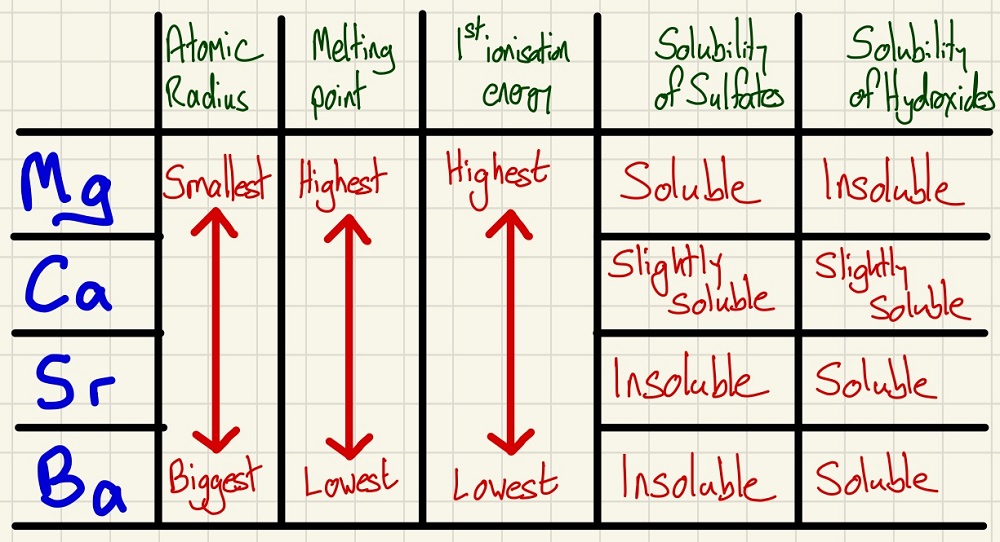
Group 2:

As you descend the group, there is an extra electron shell each time so they have a larger atomic radius. This makes the first ionisation energy also decrease as you descend, despite the nuclear charge going up, the distance to the outer electron also goes up as does the amount of shielding.

As you go down group 2, the melting points generally increase as they all have a 2+ charge attracting the ion to the delocalised electrons, however, the ions get much larger as you go down the group giving the ions a much lower charge density therefore, weaker metallic bonds.

There are patterns in solubility to learn. Sulfates get more soluble are you go up the group, whereas hydroxides are more soluble as you go down the group.

In practical use, this is why a barium meal (absorbs x-rays and can show your intestines in an X-ray) is barium sulfate. Barium is harmful if absorbed into the body, so using this form ensures that it will not dissolve and enter the blood stream. Likewise, we drink a suspension of magnesium hydroxide (milk of magnesia) to neutralise excess acid in the stomach/oesophagus. As it is insoluble, it will never lift the pH above 7.

Magnesium is very reactive and as well as being used in alloys, it can displace less reactive metals and is used in the extraction of titanium from ores, calcium carbonate is swallowed as an ant-acid and is used to catch sulfur gases from chimneys, it produces calcium sulfates which can be used in plaster. This reduces the gases that cause acid rain.
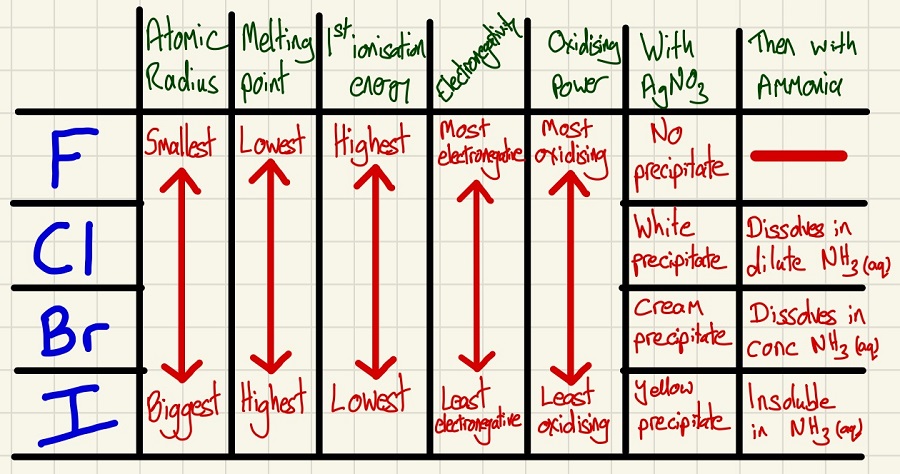
Group 7:

The halogens all have that distinctive smell and are toxic. They all work as disinfectants in different ways.

Fluorine and Chlorine are gases ate RTP, Bromine is a liquid and is used mainly in the manufacture of other chemicals and pharmaceuticals. Iodine is a grey crystalline solid that evolves a distinctive purple vapour when warmed (sublimation) and is used as an antiseptic (a solution is painted on patients before operations and it is painted onto cows' udders during milking).

The trends are very simple, as you go down the group, the atomic radius increases so the electronegativity increases. They all rely on Van der Waals' forces to keep them together and as they are proportional to mass, the melting and boiling points also increase as you go down the group.

Although we don't tend to discuss the 1st ionisation energy of a non-metal, it is important to compare properties to other groups (group 2 above). Like in group 2, the 1st ionisation energy decreases as you go down the group as the atoms' radius increases, the shielding increases so their is a weaker attraction from the protons in the nucleus to the outer electrons.

Tests:

• If you add silver nitrate to chloride ions in solution, a white precipitate is formed which redissolved in dilute ammonia solution.

• If you add silver nitrate to bromide ions in solution, a cream precipitate is formed which redissolved in concentrated ammonia solution.

• If you add silver nitrate to iodide ions in solution, a yellow precipitate is formed which will not redissolve.

REDOX relationship: Fluorine can displace chloride, bromide or iodide ions. Chlorine can displace bromide or iodide ions. Bromine can only displace iodide ions and iodide cannot displace any. Fluorine is the most powerful oxidising agent, iodine is the weakest. The inverse is also true, I- is the most powerful reducing agent and F- is the weakest.
This page was updated on: 4th November 2023
